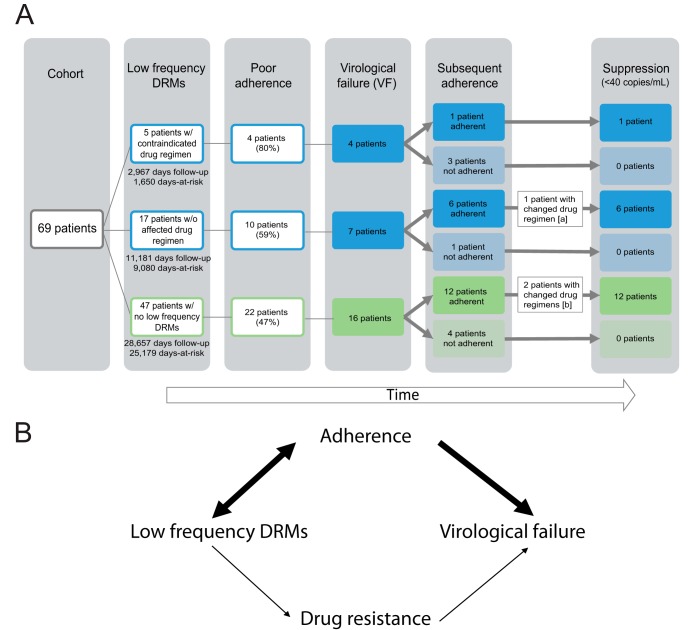
FIG. 5.
Results of clinical follow-up for patients and theoretical model. (A) Stratification of clinical outcomes in patient cohort, given the presence or absence of low-frequency DRMs associated with reduced susceptibility to their drug regimen. Adherence was defined as documentation of poor medication adherence in the clinical chart. Virological failure was defined as plasma HIV RNA level of ≥200 copies/ml at a test date more than one month after baseline. [a], Lamivudine/zidothymidine was replaced with emtricitabine/tenofovir following poor adherence to the patient’s regimen, potentially contributing to subsequent suppression. [b], Darunavir was replaced with dolutegravir in both patients’ regimens, potentially contributing to subsequent suppression. Days at risk refers to the time between sample date and censor date. Patients were censored at the date of measured virological failure or last available plasma HIV RNA quantification test. Days follow-up includes all days between the sample date and last plasma HIV RNA quantification test. (B) Low-frequency DRMs are correlated with ongoing medication adherence as a result of prior drug experience and current selective drug pressure, which is associated with virological failure regardless of drug resistance.