Corynebacterium spp. are rarely considered pathogens, but data on Corynebacterium spp.
KEYWORDS: Corynebacterium, Corynebacterium striatum, orthopedic infections, biofilms, susceptibility testing
ABSTRACT
Corynebacterium spp. are rarely considered pathogens, but data on Corynebacterium spp. as a cause of orthopedic infections are sparse. Therefore, we asked how often Corynebacterium spp. caused an infection in a defined cohort of orthopedic patients with a positive culture. In addition, we aimed to determine the species variety and the susceptibility of isolated strains to define potential treatment strategies. We retrospectively assessed all bone and joint samples that were collected between 2006 and 2015 from an orthopedic ward and that were positive for Corynebacterium spp. by culture. The isolates were considered relevant to an infection if the same Corynebacterium sp. was present in at least two samples. We found 97 orthopedic cases with isolation of Corynebacterium spp. (128 positive samples). These were mainly Corynebacterium tuberculostearicum (n = 26), Corynebacterium amycolatum (n = 17), Corynebacterium striatum (n = 13), and Corynebacterium afermentans (n = 11). Compared to the species found in a cohort of patients with positive blood cultures hospitalized in nonorthopedic wards, we found significantly more C. striatum- and C. tuberculostearicum-positive cases but no C. jeikeium-positive cases in our orthopedic cohort. Only 16 out of 66 cases (24.2%) with an available diagnostic set of at least two samples had an infection. Antibiotic susceptibility testing (AST) showed various susceptibility results for all antibiotics except vancomycin and linezolid, to which 100% of the isolates were susceptible. The rates of susceptibility of corynebacteria isolated from orthopedic samples and of isolates from blood cultures were comparable. In conclusion, our study results confirmed that a Corynebacterium sp. is most often isolated as a contaminant in a cohort of orthopedic patients. AST is necessary to define the optimal treatment in orthopedic infections.
INTRODUCTION
Orthopedic infections, including septic arthritis of a native joint, periprosthetic joint infections (PJI), and osteomyelitis, are typically treated in specialized orthopedic centers. Most of these infections are chronic, and they are difficult to diagnose and to treat due to biofilm formation (1). A definitive criterion for orthopedic infections is growth of the same pathogen in at least two diagnostic samples or the presence of a sinus tract in implant-associated infections (2, 3). The significance of a single positive sample for skin commensals, such as coagulase-negative staphylococci, Cutibacterium species (formerly Propionibacterium species), or Corynebacterium species, is not fully clear. Often, skin commensals are considered sample contaminants.
The spectrum of human infections with corynebacteria is broad, ranging from community-acquired infections, such as conjunctivitis, pharyngitis, genitourinary tract infections, prostatitis, skin and soft tissue infections, and breast abscess, to nosocomially acquired infections, such as cerebrospinal fluid shunt infections, pneumonia, intravenous catheter-related bloodstream infections, endocarditis, postsurgical infections, urinary tract infections, and peritoneal dialysis-related peritonitis (4–12). The spectrum of Corynebacterium sp. orthopedic infections has not been described so far. In this study, we report on the spectrum of Corynebacterium isolates from patients with suspected orthopedic infections and compare it with the spectrum of species isolated from patients hospitalized in nonorthopedic wards with suspected bloodstream infections. We discriminated between infections and contaminants based on clinical and microbiological criteria and present antibiotic susceptibility data to describe treatment options.
MATERIALS AND METHODS
Orthopedic study population.
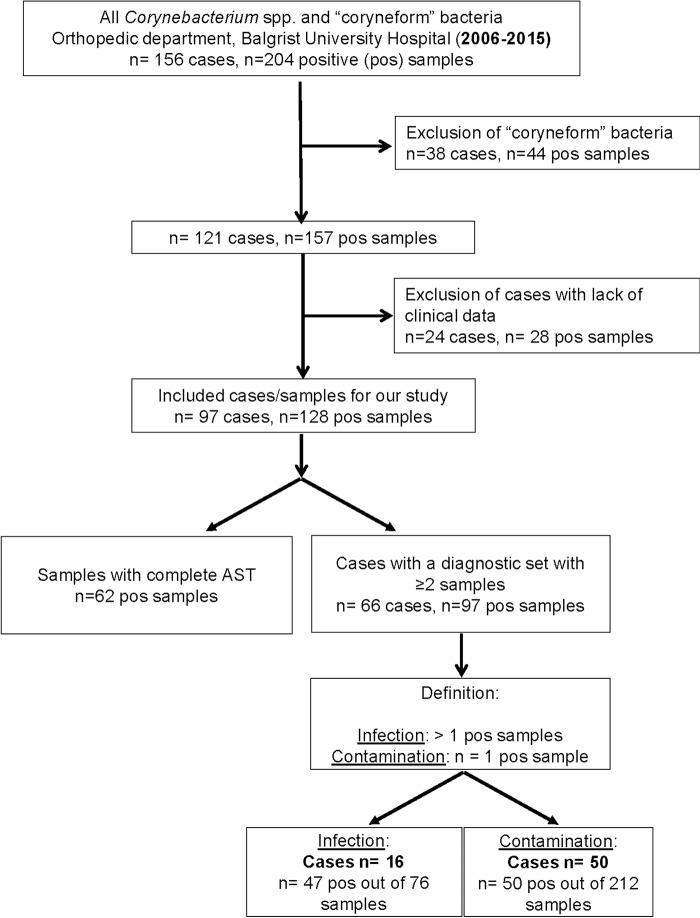
The orthopedic hospital Balgrist University Hospital in Zurich, Switzerland, is a 140-bed orthopedic center in which approximately 5,000 orthopedic procedures are performed annually. In this single-center study, we retrospectively identified patients with at least one sample from synovial fluid, deep tissue/bone, or sonication fluid from implants removed between January 2006 and December 2015 that was culture positive for Corynebacterium spp. Bacteria labeled “coryneform bacteria,” i.e., aerobic Actinomyces spp., Brevibacterium spp., Dermabacter hominis, Lactobacillus spp., Cutibacterium (Propionibacterium) spp., and other Gram-positive rods, which were not further characterized, were excluded from our analysis. The latter were found only in single samples in a small amount or in mixed cultures. Cases with a lack of clinical data were excluded for our investigation (Fig. 1).
FIG 1.
Flowchart of 97 cases and 128 positive (pos) samples in which Corynebacterium spp. were isolated, describing the variation of different species in suspected orthopedic infections, the number of samples analyzed by antibiotic susceptibility testing (AST), and the clinical characteristics of the infections.
Variation of Corynebacterium species in suspected orthopedic infections compared to bloodstream infections outside orthopedic wards.
We compared the various Corynebacterium spp. isolated from orthopedic origins with isolates recovered from blood cultures (from January 2006 to December 2015) from patients with suspected bloodstream infections hospitalized in nonorthopedic wards of the University Hospital Zurich, Zurich, Switzerland, with a wide range of medical specialties (e.g., ophthalmology, urology, gynecology, neurosurgery, vascular and heart surgery, dermatology, internal medicine, oncology).
Clinical diagnosis.
All samples from the same patient, the same hospitalization period, and the same infection site were considered one diagnostic set. The number of samples with Corynebacterium spp., the sample type, and the results of antibiotic susceptibility testing (AST) were analyzed using the database of the Institute of Medical Microbiology, University of Zurich, Zurich, Switzerland. According to the current guidelines of the Infectious Diseases Society of America (IDSA) (2), we classified a bacterium as a relevant pathogen when the same species was cultured in ≥2 samples and as a contaminant when a species was detected in only 1 of ≥2 samples (2). Cases with only one sample per diagnostic set were excluded for further analysis, as a distinction between infection and contamination was not possible in those cases. The patients’ clinical and demographic parameters and medical history, including the time of diagnosis and follow-up, were investigated using the clinical database of the orthopedic hospital and the database of the Infectious Diseases Consultation Service of the University Hospital Zurich. We grouped patients with an infection into two groups, as follows: (i) those with a monobacterial Corynebacterium sp. infection and (ii) those in whom the Corynebacterium sp. was part of a polymicrobial infection (a Corynebacterium sp. and at least another pathogen in ≥2 samples). The clinical parameters of the symptoms, such as fever, redness, swelling, and the presence of a sinus tract at the infection site, were reviewed. Patients with an infection were grouped into those with infections associated with joint arthroplasty (PJI) or another orthopedic implant (implant-associated infection), a foot or pressure ulcer with or without osteomyelitis, septic arthritis without foreign material, or a deep soft tissue infection associated with surgery. Osteomyelitis was defined when histopathology confirmed acute osteomyelitis (the presence of neutrophils) or chronic osteomyelitis (the presence of bone necrosis or sequester), when bone biopsy specimens showed the growth of a Corynebacterium sp., or when osteomyelitis was assumed by magnet resonance imaging (MRI).
In the control group with bloodstream infections, we diagnosed an infection when either ≥2 blood cultures were positive with the same Corynebacterium sp. or only 1 blood culture plus a vascular catheter was positive and sepsis criteria were fulfilled.
The study was performed in line with the current ethical guidelines and approved by the Institutional Review Board in Zurich, Switzerland (KEK no. 2016-00145).
Microbiological methods.
Prediagnostic and diagnostic processing was done as previously described (13). From 2006 to 2011, Gram-positive rods were identified by an in-house standard scheme by means of biochemical methods (14). From 2012 on, strains were identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Bruker MALDI Biotyper as described elsewhere (15). Reference database V.3.3.2.0 (3,995 entries) or later database versions were applied with a species cutoff of 1.7 (15, 16). If no or ambiguous identification was achieved by biochemical characterization or MALDI-TOF MS and a Gram-positive rod was isolated from more than one sample or from a normally sterile fluid, the identification was confirmed by 16S rRNA gene sequence analysis (17). Corynebacterium spp. that could not be assigned to the species level due to the high degree of homology of the 16S rRNA gene sequences were identified to the genus level as a Corynebacterium sp. (18).
Screening for antimicrobial susceptibility was performed by disc diffusion tests on Mueller-Hinton agar (Becton, Dickinson, Cockeysville, MD) supplemented with 5% sheep blood before 2011 and on Mueller-Hinton agar (Becton, Dickinson) supplemented with defibrinated horse blood and 20 mg/liter beta-NAD (MH-F) from 2011 on; zone diameters were interpreted according to CLSI guidelines for staphylococci as the closest surrogate for corynebacteria before 2011 (19), according to EUCAST breakpoints for staphylococci from 2011 to 2013, and according to EUCAST guidelines for corynebacteria from 2014 on (20). For this study, zone diameters obtained during the period from 2011 to 2013 were reinterpreted retrospectively according to the latest EUCAST guidelines for corynebacteria (20). MIC testing by Etest was done for relevant Corynebacterium isolates and single drugs to confirm the results of the disc diffusion tests and for strains with poor growth throughout the study period (data not shown).
Statistical analysis.
Categorical data were tested for differences using Fisher’s exact test or the chi-square test, as appropriate, whereas continuous variables were tested using Wilcoxon rank sum tests. Two-tailed P values of <0.05 were considered statistically significant.
RESULTS
Corynebacterium strains and variation.
We identified 128 Corynebacterium sp. isolates in 97 cases at the orthopedic hospital Balgrist University Hospital between 2006 and 2015 (Fig. 1). The median age of all included patients was 66 years (range, 9 to 86 years), with a predominance of men (67%); among the patients with an infection, the median age was 54.5 years (range, 25 to 83 years), and 56.3% of them were men. Most commonly, Corynebacterium tuberculostearicum (n = 26), Corynebacterium amycolatum (n = 17), Corynebacterium striatum (n = 13), or Corynebacterium afermentans (n = 11) was found as the single pathogen in 97 cases with suspected orthopedic infections (Table 1). In 13 cases, identification of Corynebacterium to the species level was not available. In two cases, two different Corynebacterium spp. were cultivated in the same diagnostic set.
TABLE 1.
Variation of Corynebacterium strains isolated from 97 patients with suspected orthopedic infections and Corynebacterium strains recovered from blood cultures from 70 patients with suspected bloodstream infectionsa
| Species | No. (%) of patients with positive orthopedic isolatesb (n = 97) |
No. (%) of patients with positive blood cultures (n = 70) |
P value | No. of orthopedic infections (n = 16) |
No. of bloodstream infections (n = 17) |
|---|---|---|---|---|---|
| C. accolens | 1 (1.0) | 1 (1.4) | 1.0 | 0 | 1 |
| C. afermentans | 11 (11.4) | 8 (11.4) | 1.0 | 0 | 0 |
| C. amycolatum | 17 (17.5) | 11 (15.7) | 0.8 | 3 | 7 |
| C. aurimucosum | 2 (2.1) | 3 (4.3) | 1.0 | 1 | 0 |
| C. diphtheriae bv. mitis | 1 (1.0) | 0 (0) | 1 | 0 | |
| C. glucuronolyticum | 1 (1.0) | 1 (1.4) | 0 | 0 | |
| C. imitans | 0 | 2 (2.9) | 0 | 0 | |
| C. jeikeium | 0 | 14 (20.0) | 0.0001 | 0 | 7 |
| C. macginleyi | 0 | 1 (1.4) | 0 | 0 | |
| C. minutissimum | 1 (1.0) | 4 (5.7) | 0.162 | 0 | 1 |
| C. mucifaciens | 1 (1.0) | 4 (5.7) | 0 | 0 | |
| C. pseudodiphtheriticum | 1 (1.0) | 0 (0) | 0 | 0 | |
| C. propinquum | 0 | 1 (1.4) | 0 | 0 | |
| C. simulans | 5 (5.2) | 2 (2.9) | 0.700 | 1 | 0 |
| C. striatum | 13 (13.4) | 2 (2.9) | 0.026 | 3 | 1 |
| C. tuberculostearicum | 26 (26.8) | 3 (4.3) | 0.001 | 3 | 0 |
| C. ureicelerivorans | 2 (2.1) | 0 (0) | 1 | 0 | |
| Corynebacterium sp. | 13 (13.4) | 13 (18.6) | 1 | 0 | |
| Polymicrobialc | 2 (2.06) | 0 | 2 | 0 |
Of the 97 patients with suspected orthopedic infections, the infection was proved in 16, and of the 70 patients with suspected bloodstream infections, the infection was proved in 17. n, total number of isolates in each category.
The most commonly isolated orthopedic strains are labeled in bold.
Isolation of two different Corynebacterium strains (one case with C. aurimucosum and C. amycolatum and one case with C. auris and C. aurimucosum).
For 31 out of the 97 cases, we could not distinguish between infection and contamination, as only one diagnostic sample was sent for microbiological analysis. Among the remaining 66 cases with ≥2 samples, we identified 16 cases (24.2%) with an infection due to a Corynebacterium sp.; in 50 cases, only one sample was positive by culture, and thus, the Corynebacterium sp. was considered a contaminant.
Of the 66 cases with ≥2 samples, the median number of analyzed samples per case was 4 (range, 2 to 11); in the cohort of 16 cases with infections due to a Corynebacterium sp., the median was 5 (range, 2 to 8), with a mean rate of positivity of 67.8% ± 28.8% (standard deviation).
Infections were associated with a native joint (n = 1, 6.3%) or prosthetic joint (PJI, n = 4, 25%), an orthopedic implant other than a joint prosthesis (n = 5, 31.3%), a deep foot or pressure ulcer (n = 4, 25%), or a deep postsurgical soft tissue infection (n = 2, 12.5%) (see Table S1 in the supplemental material). All infection sites were found in the lower extremities (feet, n = 7; knee, n = 5; hip, n = 3; gluteus, n = 1). Knee arthroplasty was involved in 75% (n = 3) of the PJI cases.
Typical inflammatory signs, including redness, pain, or swelling, were present in the majority (n = 10, 62.5%) of the infections. Among the four cases diagnosed with PJI, all presented as chronic infections with a sinus tract after a prolonged wound healing. Within the four patients with a deep ulcer diagnosis, acute osteomyelitis was additionally diagnosed.
Nine out of 16 infections (56.3%) were diagnosed as polymicrobial infections, including in the four patients with an ulcer diagnosis. In addition to corynebacteria, staphylococci, streptococci, and Enterobacteriaceae were recovered most frequently from samples with polymicrobial infections. Monobacterial infections (n = 7) were caused by either C. striatum (n = 3), C. tuberculostearicum (n = 1), C. amycolatum (n = 1), Corynebacterium ureicelerivorans (n = 1), or a Corynebacterium sp. (n = 1) which could not further be specified even after sequencing of the strain. Table S1 summarizes all mono- and polymicrobial infections grouped according to infection diagnosis.
The majority of the infections were diagnosed while the patient was already taking antibiotics (n = 9). Antibiotic treatment widely differed, on the one hand, due to the different pathogens in the mostly polymicrobial infections but also, on the other hand, due to the different antimicrobial susceptibility testing results for the isolated Corynebacterium spp.
Surgical treatment was debridement (in the presence of an implant with retention of the implant) in five cases, definitive removal of the implant in five cases, amputation in four cases, and an exchange of the implant in two cases. Since all cases with a PJI presented with a sinus tract as a sign of chronic infection, an aggressive surgical treatment was chosen from the beginning with either a two-stage exchange in two patients, definitive removal of the prosthesis (girdlestone procedure) in one patient, and amputation in one patient.
The Corynebacterium species spectrum in 97 orthopedic cases was different from the spectrum in 70 cases, from whom 86 blood samples positive by culture were collected from patients with suspected bloodstream infections. The diversity of the corynebacteria in blood cultures seemed broader, with a predominance of Corynebacterium jeikeium, which was not detected in the orthopedic samples, and with significantly fewer C. striatum and C. tuberculostearicum bacteria from blood cultures than from orthopedic cases (Table 1).
AST.
Susceptibility testing was done with 62 out of 128 (48.4%) orthopedic Corynebacterium isolates and 64 out of 70 (91.4%) blood culture isolates (Table 2). The percentages of susceptible orthopedic Corynebacterium isolates were 72% for gentamicin, 28% for penicillin, 81% for tetracycline, 100% for vancomycin, 44% for ciprofloxacin, 6% for clindamycin, 100% for linezolid, and 82% for rifampin using EUCAST breakpoints (2011 to 2015). The rates of susceptibility of the corynebacteria isolated from orthopedic samples and of isolates from blood cultures were comparable (Table 2; data for the rates are not shown). Table S2 shows the susceptibility testing results for the four Corynebacterium spp. most commonly isolated from orthopedic patients. All isolates were susceptible to vancomycin and linezolid. In contrast, penicillin resistance was observed in 78% of C. tuberculostearicum isolates, in 80% of C. amycolatum isolates, in 100% of C. striatum isolates, and in 62% of C. afermentans isolates. The different species showed variable frequencies of resistance to tetracycline, ciprofloxacin, clindamycin, rifampin, and gentamicin. Susceptibility testing of corynebacteria isolated between 2006 and 2010 was done according to CLSI recommendations. The rates of susceptibility of the isolates to gentamicin and vancomycin were similar when the results for the years 2006 to 2010 (CLSI criteria) and those for the years 2011 to 2015 (EUCAST criteria) were compared. For penicillin and tetracycline, a significantly lower rate of susceptibility was observed when EUCAST interpretive criteria (2011 to 2015) than when CLSI interpretive criteria were used (P = 0.005 and P = 0.04, respectively) (Table 2).
TABLE 2.
AST of Corynebacterium spp. isolated between 2006 and 2015
| Yr of isolation (no. of isolates) | CBPa | Sample origin | Susceptible isolates (%)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ERY | GEN | PEN | TET | VAN | CIP | CLI | LZD | RIF | |||
| 2006–2010 (26) | CLSI | Orthopedic | 35 | 85 | 65 | 100 | 100 | ND | ND | ND | ND |
| 2011–2015 (36) | EUCAST | Orthopedic | ND | 72 | 28 | 81 | 100 | 44 | 6 | 100c | 82c |
| 2006–2010 (36) | CLSI | Blood culture | 22 | 64 | 47 | 92 | 100 | ND | ND | ND | ND |
| 2011–2015 (28) | EUCAST | Blood culture | ND | 61 | 29 | 78 | 100 | 29 | 19 | 100d | 88d |
AST was performed by disc diffusion. Clinical breakpoints (CBP) from CLSI (19) were applied from 2006 to 2010, and CBP from EUCAST (20) were applied from 2011 to 2015.
ERY, erythromycin; GEN, gentamicin; PEN, penicillin; TET, tetracycline; VAN, vancomycin; CIP, ciprofloxacin; CLI, clindamycin; LZD, linezolid; RIF, rifampin; ND, not determined.
AST data for linezolid and rifampin were available for 12 and 17 isolates, respectively.
AST data for linezolid and rifampin were available for 12 and 17 isolates, respectively.
DISCUSSION
Our study identified a proven orthopedic infection due to a Corynebacterium sp. in only 24.2% of the cases from whom a Corynebacterium sp. had been isolated over a period of 10 years. We found that implant-associated infections and ulcers with or without osteomyelitis were the most common diagnosis in orthopedic patients, as previously described (21).
The Corynebacterium spp. isolated from orthopedic samples differed from the species spectrum isolated from cultured blood samples in nonorthopedic patients. We found 26 orthopedic cases with C. tuberculostearicum. This typical inhabitant of the skin is often described in association with intravascular catheter-associated infections (12) but rarely with orthopedic infections (11). C. striatum was found more frequently in orthopedic samples than in cultured blood samples (13.4% versus 2.9%) and accounted for three monomicrobial infections (two implant-associated infections and one case of septic arthritis). In the literature, C. striatum is described to be associated with foreign body-associated infections, infective endocarditis, pulmonary infections, septic arthritis, and ventilator tubes in hospital settings (12, 22, 23). C. jeikeium was recovered from 20% of our Corynebacterium-positive blood cultures but not from any of the orthopedic samples. C. jeikeium has been described to be the cause of endocarditis, bacteremia, cerebrospinal fluid shunt infections, peritoneal dialysis peritonitis, and, rarely, PJI (11, 24, 25). Biochemical characteristics and cell envelope structure are key factors for the adaptation to a certain habitat or for a predilection for a certain body site. Lipophilic corynebacteria are part of the human skin flora but can invade deep tissue, after surgery, for example. In contrast, we also described invasive infections with nonlipophilic corynebacteria (C. aurimucosum, C. simulans, and C. striatum). The pathogenesis of these infections is not yet explained.
Identification of corynebacteria to the species level is challenging and often requires 16S rRNA and rpoB gene sequencing and/or, nowadays, MALDI-TOF MS analysis, in addition to morphological and biochemical characterization (15–17, 26). Before 2012, the identification of corynebacteria to the species level by biochemical identification followed by 16S rRNA gene sequencing was restricted in the laboratory of the Insitute of Medical Microbiology of the University of Zurich to relevant Gram-positive rod isolates. In 2012, our laboratory introduced MALDI-TOF MS for the identification of bacteria; rapid and reliable identification became available for most Gram-positive rods. However, we do not exclude the possibility that we missed infections before the application of MALDI-TOF MS.
There are no clinical data available on how to best treat Corynebacterium sp. implant-associated infections. AST has to be performed on all Corynebacterium isolates causing orthopedic infections if antibiotics other than vancomycin are given (27–29). We found that only 20% to 38% of the C. tuberculostearicum, C. amycolatum, and C. afermentans isolates and none of the C. striatum isolates recovered were susceptible to penicillin. Clindamycin, ciprofloxacin, gentamicin, rifampin, and tetracycline were not regularly effective against these corynebacteria, making vancomycin the only valid empirical treatment until susceptibility tests are performed. Variable resistances were observed in different Corynebacterium spp., as already shown in the study by Reddy et al., who observed a high frequency of penicillin-resistant strains (>50%), as well as erythromycin-, clindamycin-, and gentamicin-resistant strains (30). Further studies are needed to investigate combination antibiotic treatment for the treatment of implant-associated infections, e.g., a quinolone plus rifampin, which is currently routinely used for the treatment of other Gram-positive bacterial infections, such as those caused by staphylococci (1).
Guidelines on how to perform and interpret the results of AST of corynebacteria are sparse. CLSI has provided clinical breakpoints (CBP) for broth microdilution testing of corynebacteria since 2006; EUCAST published CBPs for microdilution and disk diffusion methods in 2014. Both CLSI and EUCAST defined CBPs for all Corynebacterium spp. (for the MIC and/or zone diameter) based on small numbers of each Corynebacterium sp. or even inferred breakpoints from staphylococcal CBP (i.e., CLSI criteria for gentamicin, vancomycin, and tetracycline); in particular, EUCAST defined clinical breakpoints without defining epidemiological cutoffs (ECOFFs) separating the wild-type population from strains with resistance to a given antibiotic (http://www.eucast.org/clinical_breakpoints/, March 2017 update). The breakpoints of CLSI and EUCAST and, as a consequence, the rates of susceptible and resistant isolates may therefore differ significantly. From 2006 to 2010, we used CLSI staphylococcal breakpoints for zone diameters due to the lack of breakpoints specific for corynebacteria. Penicillin susceptibility was observed in 65% of the isolates. In contrast, applying the EUCAST breakpoints for corynebacteria resulted in only 28% susceptible isolates. This shift was mainly caused by a change of the antibiotic discs used, with the discs with 10 units of penicillin (CLSI) used previously being switched to discs with 1 unit of penicillin at present (EUCAST). It will be necessary to collect more data on corynebacteria so that breakpoints can be defined for each species individually on the basis of the distribution of the zone diameters and MICs of strains without acquired resistance.
Our observation that a Corynebacterium sp. is rarely responsible for an infection when isolated on an orthopedic ward is representative of the findings for only a single hospital and may not represent experiences at other sites. However, it is also mentioned in the report of Cazanave et al. that Corynebacterium spp. are only occasional causes of PJI as a typical orthopedic infection (11). The strength of our retrospective analysis is the long observation period of 10 years in a large orthopedic center. We might have missed some infections due to our strict definition of at least two positive diagnostic samples. In patients with foot ulcers, often only one bone biopsy specimen was taken for microbiology and histopathology, and thus, the numbers that we present are probably an underestimation. Nevertheless, our study highlights the fact that the isolation of Corynebacterium spp. from orthopedic patients rarely indicates an infection even when they are isolated from more than one sample. However, when diagnosed, Corynebacterium sp. infections are often polymicrobial. It is crucial to test the antimicrobial susceptibility of clinically relevant Corynebacterium spp. since resistance varies between different species. It remains to be investigated under which conditions corynebacteria change from a commensal to an invasive pathogen.
Supplementary Material
ACKNOWLEDGMENTS
Yvonne Achermann was supported by the academic career program Filling the Gap of the Medical Faculty of the University of Zurich.
We thank Thomas Klein and the technicians of the Institute of Medical Microbiology of the University of Zurich for expert help and assistance.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01200-18.
REFERENCES
- 1.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 2.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. 2011. New definition for periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Asten SA, La Fontaine J, Peters EJ, Bhavan K, Kim PJ, Lavery LA. 2016. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis 35:293–298. doi: 10.1007/s10096-015-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mottola C, Mendes JJ, Cristino JM, Cavaco-Silva P, Tavares L, Oliveira M. 2016. Polymicrobial biofilms by diabetic foot clinical isolates. Folia Microbiol (Praha) 61:35–43. doi: 10.1007/s12223-015-0401-3. [DOI] [PubMed] [Google Scholar]
- 6.Reece RM, Cunha CB, Rich JD. 2014. Corynebacterium minutissimum vascular graft infection: case report and review of 281 cases of prosthetic device-related Corynebacterium infection. Scand J Infect Dis 46:609–616. doi: 10.3109/00365548.2014.918650. [DOI] [PubMed] [Google Scholar]
- 7.Hong HL, Koh HI, Lee AJ. 2016. Native valve endocarditis due to Corynebacterium striatum confirmed by 16S ribosomal RNA sequencing: a case report and literature review. Infect Chemother 48:239–245. doi: 10.3947/ic.2016.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagemann JB, Essig A, Herrmann M, Liebold A, Quader MA. 2015. Early prosthetic valve endocarditis caused by Corynebacterium kroppenstedtii. Int J Med Microbiol 305:957–959. doi: 10.1016/j.ijmm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Beltran-Arroyave C, Diaz-Diaz A, Loaiza-Diaz N. 2016. Chronic osteomyelitis due to Corynebacterium striatum in a female adolescent. Rev Chilena Infectol 33:696–699. (In Spanish.) doi: 10.4067/S0716-10182016000600014. [DOI] [PubMed] [Google Scholar]
- 10.Achermann Y, Trampuz A, Moro F, Wust J, Vogt M. 2009. Corynebacterium bovis shoulder prosthetic joint infection: the first reported case. Diagn Microbiol Infect Dis 64:213–215. doi: 10.1016/j.diagmicrobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Patel R. 2012. Corynebacterium prosthetic joint infection. J Clin Microbiol 50:1518–1523. doi: 10.1128/JCM.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funke G, von Graevenitz A, Clarridge JE III, Bernard KA. 1997. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev 10:125–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bossard DA, Ledergerber B, Zingg PO, Gerber C, Zinkernagel AS, Zbinden R, Achermann Y. 2016. Optimal length of cultivation time for isolation of Propionibacterium acnes in suspected bone and joint infections is more than 7 days. J Clin Microbiol 54:3043–3049. doi: 10.1128/JCM.01435-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Graevenitz A, Funke G. 1996. An identification scheme for rapidly and aerobically growing gram-positive rods. Zentralbl Bakteriol 284:246–254. doi: 10.1016/S0934-8840(96)80100-9. [DOI] [PubMed] [Google Scholar]
- 15.Schulthess B, Bloemberg GV, Zbinden R, Böttger EC, Hombach M. 2014. Evaluation of the Bruker MALDI Biotyper for identification of Gram-positive rods: development of a diagnostic algorithm for the clinical laboratory. J Clin Microbiol 52:1089–1097. doi: 10.1128/JCM.02399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alatoom AA, Cazanave CJ, Cunningham SA, Ihde SM, Patel R. 2012. Identification of non-diphtheriae Corynebacterium by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 50:160–163. doi: 10.1128/JCM.05889-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosshard PP, Abels S, Zbinden R, Böttger EC, Altwegg M. 2003. Ribosomal DNA sequencing for identification of aerobic gram-positive rods in the clinical laboratory (an 18-month evaluation). J Clin Microbiol 41:4134–4140. doi: 10.1128/JCM.41.9.4134-4140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodfellow MPA, Kämpfer P, Busse H-J, Trujillo ME, Suzuki K-I, Ludwig W, Whitman WB. 2012. The actinobacteria In Bergey’s manual of systematic bacteriology, vol. 5A Springer, New York, NY. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.EUCAST. 2014. Breakpoint tables for interpretation of MICs and zone diameters, version 4.0. European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org.
- 21.Arciola CR, An YH, Campoccia D, Donati ME, Montanaro L. 2005. Etiology of implant orthopedic infections: a survey on 1027 clinical isolates. Int J Artif Organs 28:1091–1100. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Martinez L, Suarez AI, Winstanley J, Ortega MC, Bernard K. 1995. Phenotypic characteristics of 31 strains of Corynebacterium striatum isolated from clinical samples. J Clin Microbiol 33:2458–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandenburg AH, van Belkum A, van Pelt C, Bruining HA, Mouton JW, Verbrugh HA. 1996. Patient-to-patient spread of a single strain of Corynebacterium striatum causing infections in a surgical intensive care unit. J Clin Microbiol 34:2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezaei Bookani K, Marcus R, Cheikh E, Parish M, Salahuddin U. 2018. Corynebacterium jeikeium endocarditis: a case report and comprehensive review of an underestimated infection. IDCases 11:26–30. doi: 10.1016/j.idcr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao CT, Huang JW, Yen CJ. 2013. A rare and under-recognized pathogen in peritoneal dialysis peritonitis: Corynebacterium jeikeium. Perit Dial Int 33:580–581. Canada. doi: 10.3747/pdi.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khamis A, Raoult D, La Scola B. 2004. rpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol 42:3925–3931. doi: 10.1128/JCM.42.9.3925-3931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagrou K, Verhaegen J, Janssens M, Wauters G, Verbist L. 1998. Prospective study of catalase-positive coryneform organisms in clinical specimens: identification, clinical relevance, and antibiotic susceptibility. Diagn Microbiol Infect Dis 30:7–15. doi: 10.1016/S0732-8893(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez JS, Peris BM, Guirao GY, Zufiaurre NG, Bellido JM, Hernandez MS, Rodriguez JG. 2003. In vitro activity of newer antibiotics against Corynebacterium jeikeium, Corynebacterium amycolatum and Corynebacterium urealyticum. Int J Antimicrob Agents 22:492–496. doi: 10.1016/S0924-8579(03)00121-3. [DOI] [PubMed] [Google Scholar]
- 29.Soriano F, Zapardiel J, Nieto E. 1995. Antimicrobial susceptibilities of Corynebacterium species and other non-spore-forming gram-positive bacilli to 18 antimicrobial agents. Antimicrob Agents Chemother 39:208–214. doi: 10.1128/AAC.39.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BS, Chaudhury A, Kalawat U, Jayaprada R, Reddy G, Ramana BV. 2012. Isolation, speciation, and antibiogram of clinically relevant non-diphtherial corynebacteria (diphtheroids). Indian J Med Microbiol 30:52–57. doi: 10.4103/0255-0857.93033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.