Figure 1.
Reversible Streptavidin Binding of Synthetic Surface Expressing Biotin Mimics
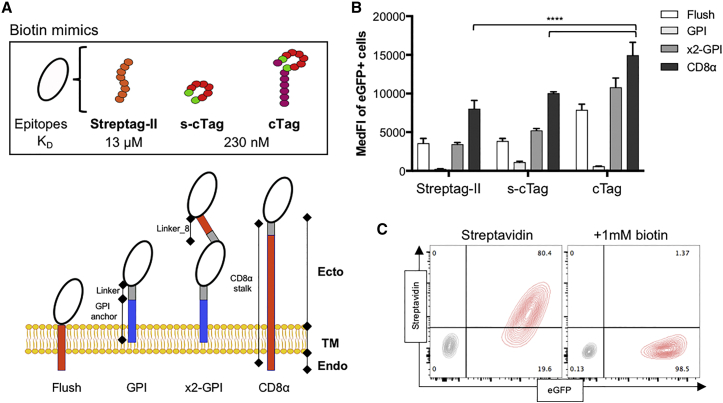
(A) Schematic diagrams of the chosen mimics with their reported dissociation constants (KD). Four surface expression structures were cloned, and their architectures are represented: (1) Flush, consisting of a CD8-derived transmembrane (TM) and endodomain (Endo); (2) GPI, consisting of GPI anchor sequence (25 amino acids [aa]), which leads to the addition of GPI at the anchor sequence, with a serine-glycine linker (6 aa); (3) x2-GPI, consisting of 2 copies of the epitopes’ open reading frame (ORF) separated by a serine-glycine linker with the first 14 aa of the CD8α stalk ectodomain (Ecto; Linker_CD8), on a GPI anchor sequence; (4) CD8α, consisting of the CD8α stalk comprising the ecto-, transmembrane, and endodomains of the human CD8α molecule, with a serine-glycine linker. All peptides were cloned into a retroviral plasmid termed “SFG,” derived from the Moloney murine leukemia virus (MoMLV), upstream of the EGFP marker gene expressed by an internal ribosome entry site (IRES). (B) 293T cells were transiently transfected with all cloned constructs and stained with APC-conjugated streptavidin 48 hr later. The median fluorescence intensity (MedFI) of streptavidin binding of EGFP-positive cells are presented in a graph indicating ±SD of three independent transient expression experiments; ****p < 0.0001. (C) Negative control 293T (gray population) and cTag8-expressing 293T cells co-expressing EGFP (cTag8 293T, red population) were first stained with streptavidin conjugated to APC, and samples were analyzed by flow cytometry. Samples were then washed and incubated with 1 mM biotin for 1 hr at room temperature and analyzed by flow cytometry. Results are presented as overlaid contour plots before (Streptavidin) and after (1 mM biotin) biotin addition.