Figure 3.
Streptavidin-Mediated Complete Capture of cTag8-Modified LVs
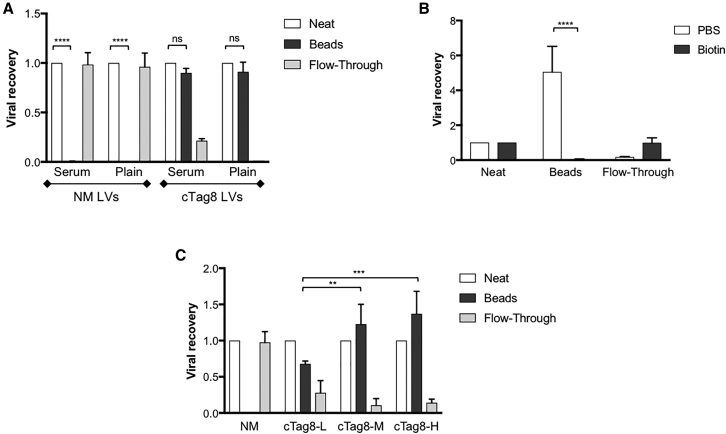
Transiently produced LVs from 293T and cTag8 293T cells, termed NM LVs and cTag8 LVs, respectively, were incubated with streptavidin magnetic beads. Dynabeads were immobilized by magnetic capture, and flow-through fractions were collected. Streptavidin Dynabeads were then washed 4 times with cold PBS and resuspended in cold medium, in the same volume as starting viral supernatants. Viral titers (IU/mL) of all fractions: (1) crude (Neat), (2) re-suspended magnetic bead (Beads), and (3) post-capture incubation flow-through (Flow-Through) fractions were determined by infectivity assay on 293T cells. (A) NM LVs and cTag8 LVs were produced in either serum-supplemented IMDM (serum) or serum-free DMEM (plain) medium. Viral supernatants were then subjected to capture methodology for 2 hr at 4°C. (B) Streptavidin Dynabeads were pre-treated either with plain or 15 mM biotin-supplemented PBS for 1 hr at room temperature. After 3 washes with PBS, pre-treated beads were incubated with cTag8 LVs in serum-free media for 1 hr at room temperature. (C) LVs were produced in serum-free media from low (L), medium (M), and high (H) cTag8-expressing 293T cells transiently. Along with control NM LVs, all viral supernatants were incubated with streptavidin Dynabeads for 1 hr at room temperature. All values presented represent viral recovery of each fraction compared to corresponding total vector input. Data are plotted ± SD of triplicate determinations. **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001; ns, non-significant.