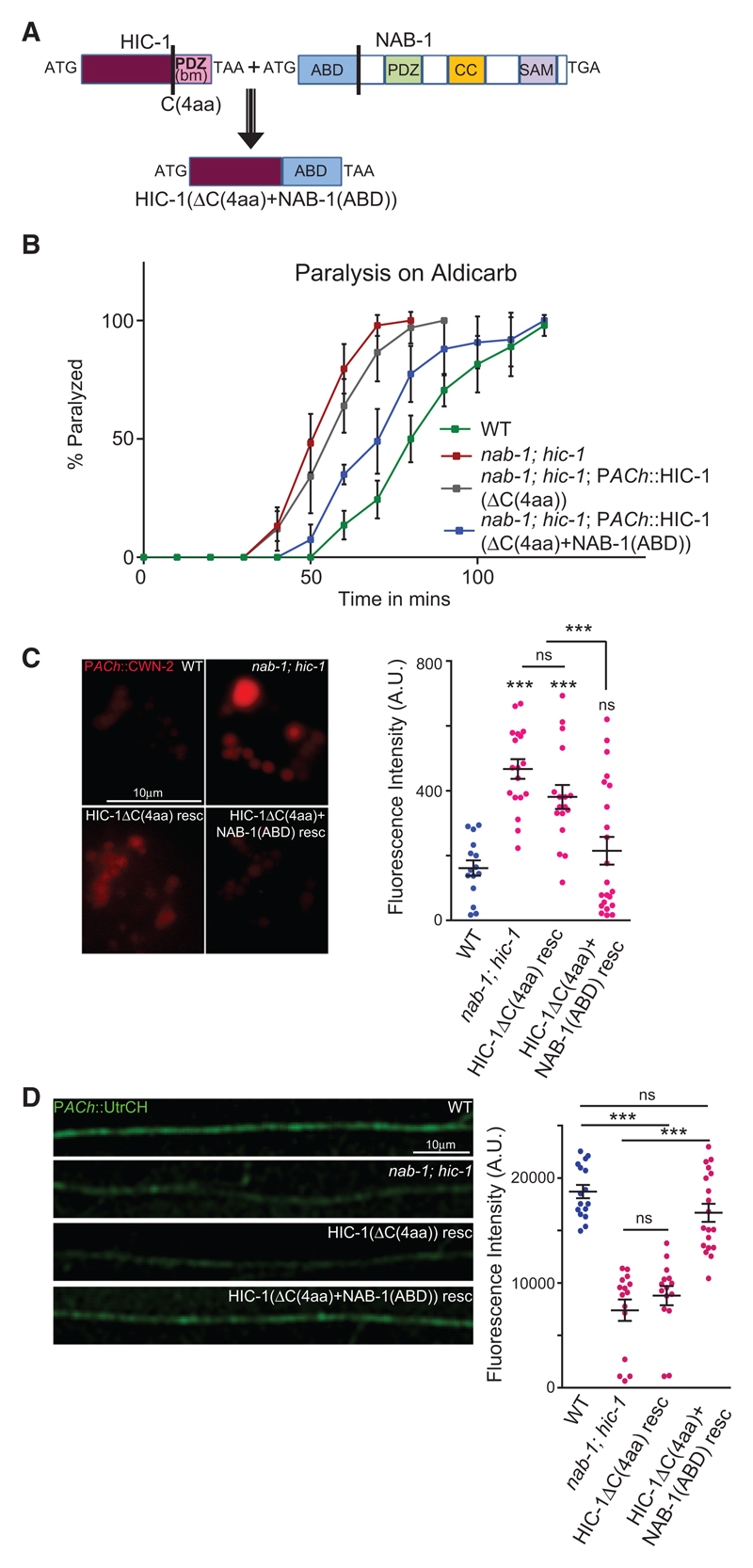
Figure 7. The ABD of Neurabin Linked to HIC-1 Is Sufficient to Rescue the NMJ Defects of the nab-1; hic-1 Double Mutants.
(A) Schematic of the fusion construct HIC-1(ΔC(4aa)+NAB-1(ABD)). The C-terminal four amino acids were deleted from HIC-1, and the ABD of NAB-1 was added in frame with the above HIC-1 construct. The domains of NAB-1 are shown (Chia et al., 2012).
(B) Time course paralysis on Aldicarb for the following strains: WT, nab-1; hic-1, nab-1; hic-1; PACh::HIC-1(ΔC(4aa)), and nab-1; hic-1; PACh::HIC-1(ΔC (4aa)+NAB-1(ABD)). The experiment is performed six times, and the total number of animals used in each experiment was 120 (20 animals/trial) for each genotype.
(C) Representative images and quantitation of coelomocyte fluorescence intensity from PACh::Wnt/CWN-2::mCherry. WT (n = 15), nab-1;hic-1 (n = 18), nab-1; hic-1; PACh::HIC-1(ΔC(4aa)) (n = 17), and nab-1; hic-1; PACh::HIC-1(ΔC(4aa)+NAB-1(ABD)) (n = 22).
(D) Representative images and quantitation of the DNC of animals expressing GFP-UtrCH as a transgene in cholinergic neurons. The genotypes used in this experiment were WT (n = 16), nab-1; hic-1 (n = 15), nab-1; hic-1; PACh::HIC-1(ΔC(4aa)) (n = 15), and nab-1; hic-1; PACh::HIC-1(ΔC(4aa)+NAB-1(ABD)) (n = 19).
In (C) and (D), p values were calculated using one-way ANOVA and Bonferroni’s multiple-comparison test. ***p < 0.001; ns, not significant. Data are represented as mean ± SEM.