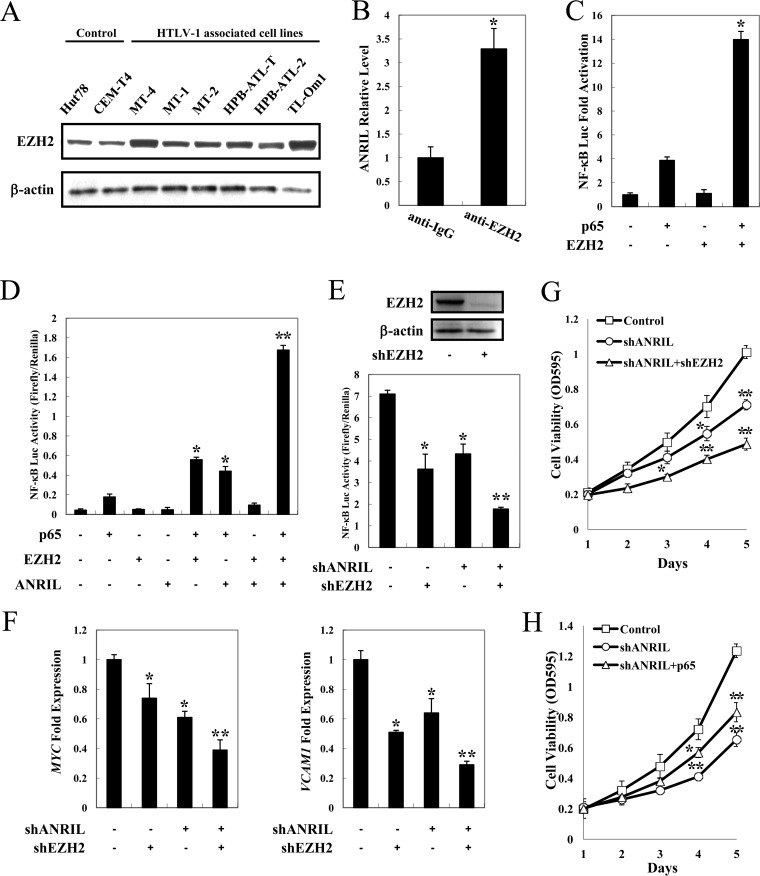
FIG 6.
ANRIL/EZH2 activates the NF-κB pathway. (A) Overexpression of EZH2 in ATL. Total proteins were extracted from ATL and HTLV-1-immortalized cell lines. The level of EZH2 protein was analyzed by immunoblotting. (B) ANRIL-associated EZH2 in vivo. Shown are data for radioimmunoprecipitation analysis of the association of endogenous ANRIL RNA and EZH2 protein in HPB-ATL-T cells. (C) EZH2 enhances p65-induced NF-κB activation. Jurkat cells were cotransfected with κB-Luc, phRL-TK, pCMV-p65, and lenti-Myc-EZH2. After 48 h, luciferase activity was measured. (D) ANRIL and EZH2 synergistically reinforce p65-mediated NF-κB activation. Jurkat cells were cotransfected with κB-Luc, phRL-TK, pCSII-CMV-ANRIL, lenti-Myc-EZH2, and pCMV-p65. After 48 h, the cells were harvested, and luciferase activity was determined. (E) Silencing of EZH2 expression suppresses NF-κB signaling in ANRIL knockdown cells. HPB-ATL-T cells were transfected with a recombinant lentivirus expressing the pLKO-shANRIL vector with or without pLKO-shEZH2. After puromycin selection, the expression levels of EZH2 and β-actin were analyzed by immunoblotting (top), and the cells were harvested and analyzed for luciferase activity (bottom). (F) Suppressed expression of selected NF-κB target genes in ANRIL and EZH2 knockdown HPB-ATL-T cells. The levels of MYC and VCAM1 mRNAs were analyzed by real-time PCR. (G) EZH2 silencing synergizes with ANRIL knockdown to inhibit cell viability. HPB-ATL-T cells were transfected with a recombinant lentivirus expressing the pLKO-shANRIL vector together with pLKO-shEZH2. After puromycin selection, cell growth was detected by an MTT assay. (H) Overexpression of p65 rescues cell proliferation inhibition by ANRIL silencing. HPB-ATL-T cells were transfected with a recombinant lentivirus expressing the pLKO-shANRIL vector together with pCSII-CMV-p65. After puromycin selection, cell growth was detected by an MTT assay. *, P < 0.05; **, P < 0.01.