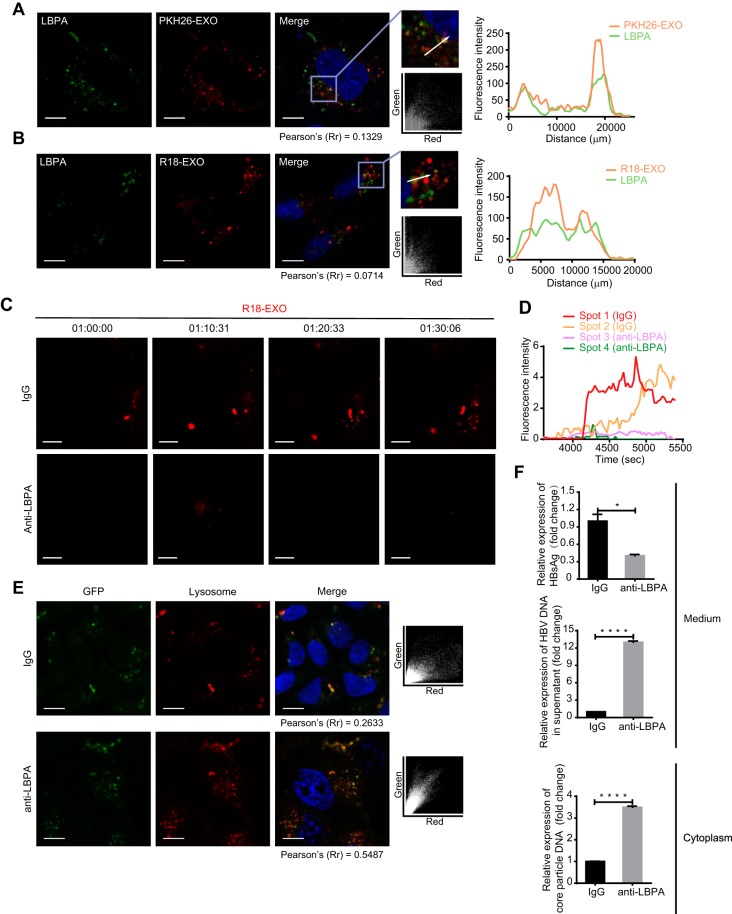
FIG 6.
LBPA is required for exosome fusion and cargo uncoating. (A) Accumulation of PKH26-labeled exosomes in LBPA-rich vacuoles. Colocalization of PKH26 (red) with LBPA (green) was analyzed as described in the text. Scale bar: 10 µm. (B) Membrane fusion signals of dequenching R18-exosomes colocalized with LBPA. Colocalization of dequenching signals (red) with LBPA (green) was analyzed as described in the text. Scale bars: 10 µm. (C) Inhibition of exosome fusion by antibodies against LBPA. Fusion spots of dequenching R18-exosomes in HepG2 cells pretreated with 50 µg/ml of anti-LBPA or anti-IgG overnight were tracked and photographed at the indicated time points. Scale bars: 5 µm. (D) Time-intensity profiles of R18 fluorescence of four representative dequenching spots in panel C. (E) Increase in colocalization of the exosomal cargo GFP with lysosomes after exposure to antibodies against LBPA. HepG2 cells pretreated with anti-LBPA or anti-IgG overnight were incubated with GFP-carrying exosomes in the presence of LysoTracker. Colocalization of GFP (green) with lysosomes (red) was analyzed via scatterplots and Pearson’s correlation coefficients. Scale bars: 10 µm. (F) HepG2.2.15 cells were pretreated with anti-IgG or anti-LBPA overnight. The level of HBsAg in the culture medium (supernatant) was detected by ELISA. HBV DNA in the culture medium and intracellular core particle DNA were quantified by qPCR. The error bars indicate the SD. *, P < 0.05; ****, P < 0.0001 (Student’s t test). The data are representative of those from three independent experiments.