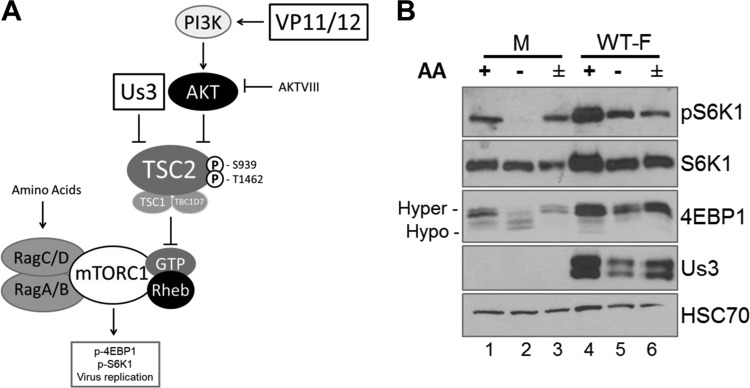
FIG 1.
Sensitivity of mTORC1 activation to AA insufficiency in uninfected and HSV-1-infected cells. (A) Cartoon illustrating how HSV-1 manipulates mTORC1 activation in virus-infected cells. The HSV-1-encoded Ser/Thr kinase Us3 enforces mTORC1 activation during infection by phosphorylating TSC2 residues S939 and T1462, the same residues targeted by Akt. This phosphorylation event inhibits TSC Rheb-GAP activity, allowing Rheb-GTP to activate mTORC1. VP11/12, which is encoded by the HSV-1 UL46 gene, stimulates PI 3-kinase (PI3K) to activate Akt. Akt inhibitor VIII (AKTVIII) is a small molecule that specifically inhibits Akt. Activation of mTORC1 in uninfected cells requires amino acid sufficiency. By promoting assembly of GTP-bound RagA/B with GDP-bound RagC/D, amino acids stimulate binding of mTORC1 to the RAG complex on the lysosomal membrane surface and position mTORC1 proximal to its activator Rheb. Once activated, mTORC1 phosphorylates substrates, including 4E-BP1 and p70S6K1, to stimulate productive virus replication. (B) NHDFs growth arrested by serum deprivation were mock infected (M) or infected with the WT HSV-1 F strain (WT-F). At 9 hpi, cells were incubated in AA-free RPMI 1640 for 50 min (−), incubated in AA-free RPMI 1640 for 50 min followed by a 30-min restimulation in AA-replete RPMI 1640 (±), or left in AA-replete DMEM for 50 min (+). Total protein was isolated and analyzed by immunoblotting using the indicated antibodies. Migration of hyperphosphorylated (Hyper-) and hypophosphorylated (Hypo-) 4E-BP1 is indicated to the left of the panel. HSC70 serves as a loading control. pS6K1, S6K1 phosphorylated at Thr389.