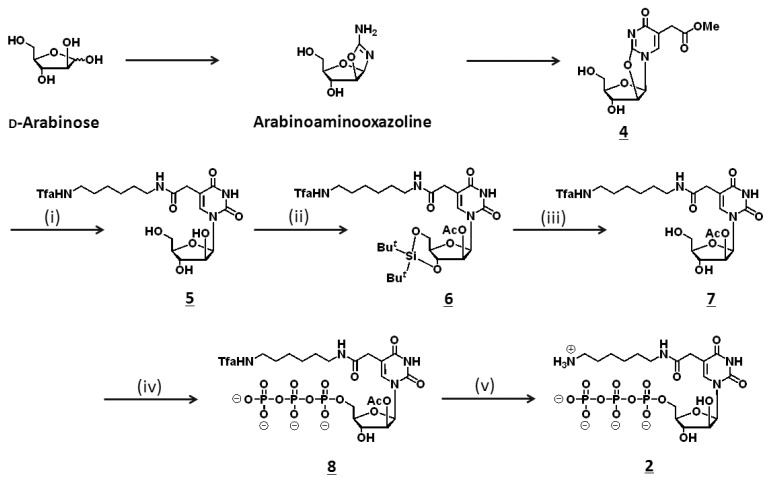
Scheme 1.
Synthesis of thymidine analogue 2.
Reagents and Conditions: (i) hexamethylenediamine, DMAP, 50°C, 1 h; 2N NaOH aq., r.t., 36 h; trifluoroacetic acid ethylester, TEA, 18 h; (ii) di-tert-butylsilyl-bis(trifluoro- methanesulfonate), DMF, 0 °C, 1 h; imidazole, r.t., 1 h; pyridine, acetic anhydride, r.t., 1 h; (iii) pyridinium poly(hydrogenfluoride), r.t., overnight; (iv) POCl3, N,N,N′,N′-tetramethyl-1,8-naphthalendiamine, trimethyl phosphate, 0°C, 45 min; n-tributylamine pyrophosphate, DMF, r.t., 1 h; (v) 4N NH4OH aq., r.t., 2 h.