Abstract
Logarithms of stability constants, log K1 and log β2, of the first transition series metal mono- and bis-complexes with any of four aliphatic amino acids (glycine, alanine, valine and leucine) decrease monotonously with third order valence connectivity index, 3χv, from Cu2+ to Mn2+. While stability of the complexes with the same metal is linearly dependent on 3χv, stability constants of Mn2+, Fe2+, Co2+, and Ni2+complexes with the same ligand show a quadratic dependence on 3χv. As Cu2+ complexes deviate significantly from quadratic functions, models for the simultaneous estimation of the stability constants, yielding r = 0.999 (S.E. = 0.05) and r = 0.998 (S.E. = 0.11), for log K1 and log β2, respectively, were developed only for Mn2+, Fe2+, Co2+, and Ni2+ complexes with amino acids.
Keywords: amino acid chelates, topological indices, regression models
1. Introduction
The Irving-Williams order of stability of bivalent transition metal complexes (Mn2+<Fe2+<Co2+ <Ni2+<<Cu2+>Zn2+) [1,2] is an empirical rule well known to every chemist, but it was rarely used for the quantitative prediction of stability constants. Cannon developed an interpolation formula [3] to predict stability constants of chromium(II) complexes from the constants of copper(II), manganese(II), and zinc(II), but it was later found to be in no way better than similar formulas based on one variable, namely stability constants of copper(II), or merely protonation constant of the ligand [4].
In our systematic attempt to develop regression models based on the third order valence connectivity index (3χv) for the prediction of stability constants of coordination compounds [5,6], we were concerned mostly with the copper(II) and nickel(II) chelates. We developed models not only for the complexes of the same metal, but also the simultaneous prediction of stability constants of copper(II) and nickel(II). This was done by introducing an indicator variable, i.e. assuming that the regression lines for the two metals have the same slope [7,8].
Connectivity, as well as other topological indices [9,10], have found wide range of application in all fields of chemisty [11,12,13,14], but they were not used for the prediction of stability constants of coordination compounds before the appearance of our pioneering work in 1999 [15]. Even now, many chemists are reluctant to use such a simple method, as models based on topological indices really are, to solve such a complex problem as prediction of stability of coordination compounds.
However, regression models based on valence connectivity index of the third order are capable of predicting stability constants with an error usually less than 0.3 log K units, and were even successfully used for the evaluation of experimental data obtained by two different electrochemical methods, potentiometry and voltammetry [16]. The regression models were successfully checked on complexes of α-amino acids and their N-alkylated derivatives [16,17], amines [17,18,19] and smaller peptides from dipeptides to pentapeptides [7,20]. Also, we recently applied our model to cadmium(II) complexes with amino acids [21].
The aim of this paper is to make our regression models based on 3χv index more general, i.e. to develop models that would discriminate not only ligands, but metals as well. As complexes of the metals from Irving-Williams order seems ideal for this purpose, we choose stability constants of their mono-
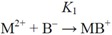 |
(1) |
and bis-complexes
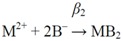 |
(2) |
with aliphatic α-amino acids (M stays for metal and B for ligand).
2. Methods
2.1. Calculation of topological indices
We calculated topological indices using a program system E-DRAGON, developed by Todeschini and coworkers [22,23], which is capable of yielding 119 topological indices in a single run, along with many other molecular descriptors [24,25]. Connectivity matrices were constructed with the aid of the Online SMILES Translator and Structure File Generator [26]. All models were developed by using 3χv index (the valence molecular connectivity index of the 3rd order), which was defined as [27,28,29]:
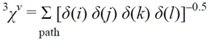 |
(3) |
where δ(i), δ(j), δ(k), and δ(l) are weights (valence values) of vertices (atoms) i, j, k, and l making up the path of length 3 (three consecutive chemical bonds) in a vertex-weighted molecular graph.Valence value, δ(i), of a vertex i is defined by:
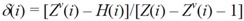 |
(4) |
where Zv(i) is the number of valence electrons belonging to the atom corresponding to vertex i, Z(i) is its atomic number, and H(i) is the number of hydrogen atoms attached to it. For instance, δ values for primary, secondary, tertiary and quaternary carbon atoms are 1, 2, 3, and 4, respectively; for oxygen in the OH group it equals 5, and for NH2 group δ(N) = 3. It has to be pointed out that 3χv is only a member of the family of valence connectivity indices nχv, which differ between each other by the path length, i.e. the number of δ´ sin the summation term, Equation (3).
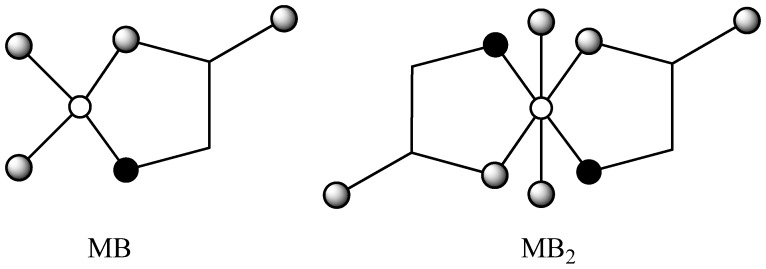
The 3χv indices for all mono- and bis-complexes were calculated from the graph representations of the aqua complexes with two water molecules (Figure 1), assuming that metal in mono-complexes is tetracoordinated, and in bis-complexes hexacoordinated [17,30].
Figure 1.
The graph representations for metal(II) mono- (MB) and bis-complex (MB2) with glycine. Heteroatoms are marked with  (M),
(M),  (N), and
(N), and  (O).
(O).
2.2. Regression calculations
Regression calculations, including the leave-one-out procedure of cross validation, cv, were done using the CROMRsel program [31]. The standard error of cross validation estimate is defined as:
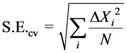 |
(5) |
where ΔX and N denotes cv residuals and the number of reference points, respectively.
2.3. Stability constants selection
Because of huge variations between experimental stability constants it was important that selected constants were measured under the same conditions (ionic strength, temperature), and preferably in the same laboratory. It was a bit surprising to find out that the most consistent constants were also the oldest, determined in 1950s. They were measured at t = 25 °C, and I = 0.01 or I → 0 mol L−1. If more than one experimental value was referred for a complex, we used mean value in further calculations. Moreover, lack of constants measured at these conditions forced us to include three constants, for Fe(Glycine), Fe(Glycine)2 and Fe(Valine), measured at t = 20 °C, I = 0.01 mol L−1. Unfortunately, appropriate log K1 andlog β2 values for Ni2+ complexes with valine were not found in the literature.
3. Results and Discussion
In this paper we tried to reproduce stability constants of chelates with bivalent metals constituting Irving-Williams order, whose stability grows monotonously (from Mn2+ to Cu2+), by means of valence connectivity index of the 3rd order, 3χv. Therefore, experimental values of stability constants of the metal complexes with four aliphatic α-amino acids (glycine, alanine, valine and leucine) were taken from the literature, Table 1.
Table 1.
Experimental stability constants for metal(II) chelates with α-amino acids.
| Metal/Ligand | log K1 | log β2 | References |
|---|---|---|---|
| Cu/Glycine | 8.57 | 15.63 | [32,33,34,35] |
| Ni/Glycine | 6.15 | 11.15 | [32,33] |
| Co/Glycine | 5.09 | 9.10 | [32,33] |
| Fe/Glycine | 4.30 | 7.80 | [36] |
| Mn/Glycine | 3.55 | 6.63 | [32,33] |
| Cu/Alanine | 8.41 | 15.21 | [32,33,37,38] |
| Ni/Alanine | 5.96 | 10.66 | [33] |
| Co/Alanine | 4.83 | 8.55 | [32,33,39] |
| Fe/Alanine | 7.30 | [39] | |
| Mn/Alanine | 3.13 | 6.05 | [32,33] |
| Cu/Valine | 7.93 | 14.45 | [32] |
| Co/Valine | 4.57 | 8.24 | [32] |
| Fe/Valine | 6.80 | [39] | |
| Mn/Valine | 2.84 | 5.56 | [32] |
| Cu/Leucine | 7.89 | 14.34 | [32] |
| Ni/Leucine | 5.62 | 10.18 | [40] |
| Co/Leucine | 4.52 | 8.16 | [32,40] |
| Mn/Leucine | 2.78 | 5.45 | [32] |
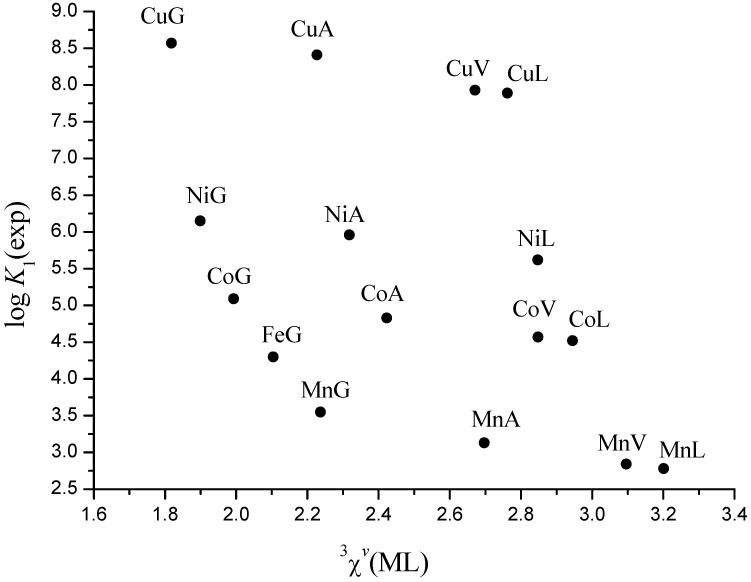
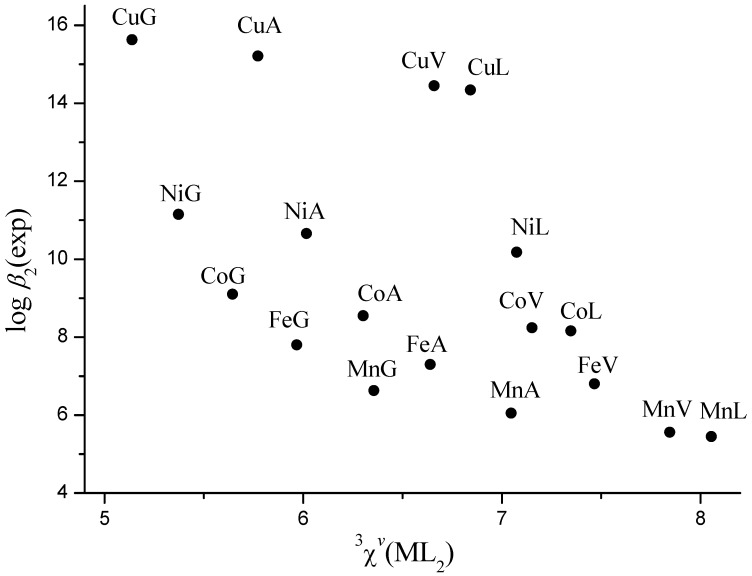
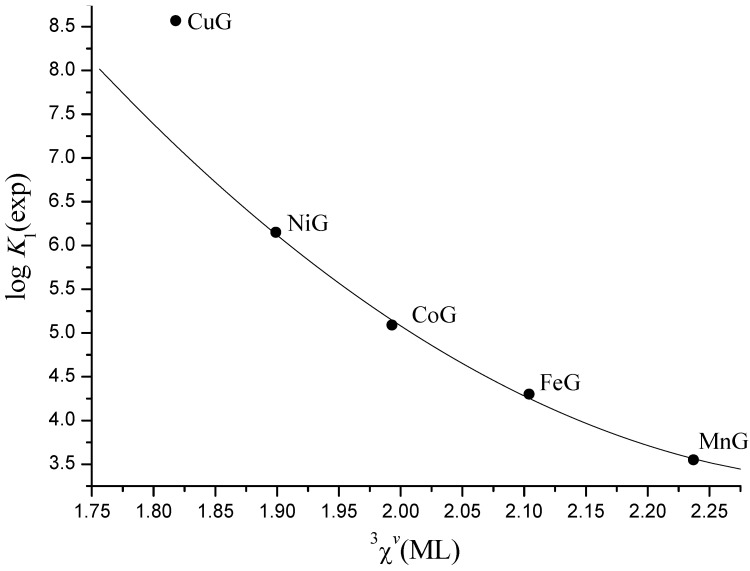
It is evident (Figure 2 and Figure 3), that stability constants of the complexes with the same metal decrease linearly with 3χv from glycine to leucine, as we have previously shown [17,30]. Figure 2 and Figure 3 also show that stability of mono- and bis-complexes decreases monotonously from Cu2+ to Mn2+ for complexes with the same ligand. However, the decrease is much more pronounced between Cu2+ and Ni2+ because Cu(II) is the strongest Lewis acid in Irwing-Williams order, and its complexes are therefore unusually stable [2]. Consequently, stability constants of Mn2+, Fe2+, Co2+, and Ni2+ complexes show quadratic dependence on 3χv, but Cu(II) complexes considerably deviate from it (Figure 4).
Figure 2.
Experimental values of log K1 vs. connectivity 3χv index for Mn2+, Fe2+, Co2+, Ni2+, and Cu2+ complexes with glycine (G), alanine (A), valine (V) and leucine (L).
Figure 3.
Experimental values of log β2 vs. connectivity 3χv index for Mn2+, Fe2+, Co2+, Ni2+, and Cu2+ complexes with glycine (G), alanine (A), valine (V) and leucine (L).
Figure 4.
Quadratic dependence of log K1 of Mn2+, Fe2+, Co2+, and Ni2+ complexes with glycine (G) on connectivity index 3χv.
Bearing this in mind we developed models for the simultaneous estimation of stability constants of Mn2+, Fe2+, Co2+ and Ni2+ complexes:
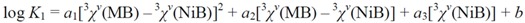 |
(6) |
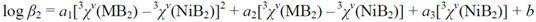 |
(7) |
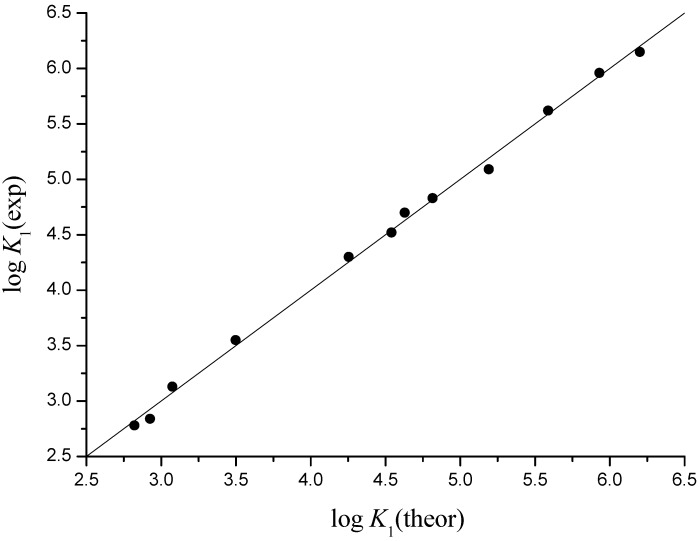
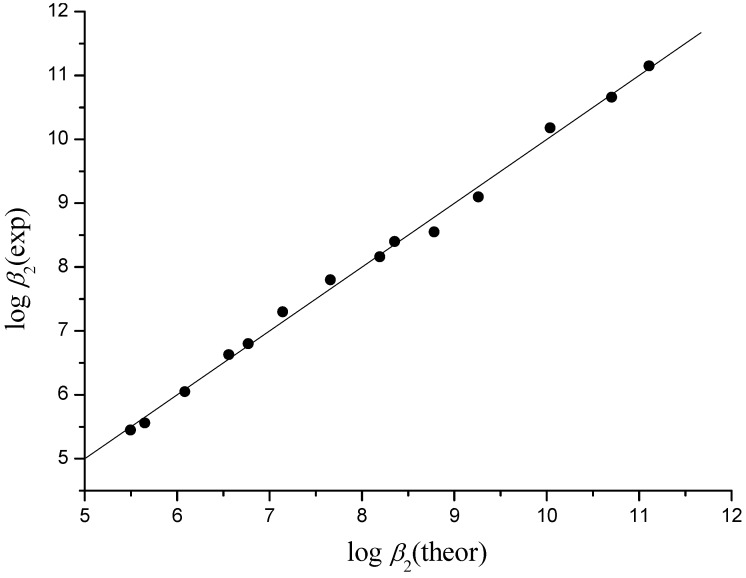
where 3χv(NiB) and 3χv(NiB2), stand for normalization along x axis in the first two terms, and for normalization along y axis in the third terms. Models gave standard error of cross validation S.E.cv = 0.08 and 0.15, and maximal cv error of 0.13 and 0.29 for log K1 and log β2, respectively (Table 1, Table 2 and Table 3, Figure 5 and Figure 6).
Table 2.
Regression models for the estimation of the stability constants of mono- and bis-complexes.
| Eq. | N | Dependentvariable | Regression coefficients | Intercept (S.E.) | r | S.E. | S.E.cv | ||
|---|---|---|---|---|---|---|---|---|---|
| a1(S.E.) | a2(S.E.) | a3(S.E.) | |||||||
| (6) | 12 | log K1 | 12.2(14) | −12.10(55) | −0.676(46) | 7.49(12) | 0.999 | 0.05 | 0.08 |
| (7) | 14 | log β 2 | 3.10(33) | −7.66(36) | −0.646(52) | 14.58(33) | 0.998 | 0.11 | 0.15 |
Table 3.
Theoretical (cross validated) stability constants for metal(II) chelates with α-amino acids, and their 3χv indices.
| Metal/Ligand | log K1 (cv) | log β2 (cv) | 3χv(MB) | 3χv(MB2) |
|---|---|---|---|---|
| Ni/Glycine | 6.21 | 11.11 | 1.90 | 5.37 |
| Co/Glycine | 5.18 | 9.25 | 1.99 | 5.65 |
| Fe/Glycine | 4.24 | 7.65 | 2.10 | 5.97 |
| Mn/Glycine | 3.51 | 6.58 | 2.24 | 6.36 |
| Ni/Alanine | 5.93 | 10.69 | 2.32 | 6.02 |
| Co/Alanine | 4.79 | 8.76 | 2.42 | 6.30 |
| Fe/Alanine | 7.13 | 2.55 | 6.64 | |
| Mn/Alanine | 3.09 | 6.10 | 2.70 | 7.05 |
| Ni/Valine | 2.75 | 6.89 | ||
| Co/Valine | 4.59 | 8.32 | 2.85 | 7.15 |
| Fe/Valine | 6.74 | 2.96 | 7.47 | |
| Mn/Valine | 2.91 | 5.64 | 3.10 | 7.85 |
| Ni/Leucine | 5.57 | 10.01 | 2.85 | 7.08 |
| Co/Leucine | 4.50 | 8.16 | 2.95 | 7.35 |
| Mn/Leucine | 2.81 | 5.48 | 3.20 | 8.06 |
Figure 5.
Experimental vs. theoretical (fit) log K1 for Mn2+, Fe2+, Co2+, and Ni2+ complexes with glycine, alanine, valine and leucine; r = 0.999, S.E.cv = 0.08.
Figure 6.
Experimental vs. theoretical (fit) log β2 for Mn2+, Fe2+, Co2+, and Ni2+ complexes with glycine, alanine, valine and leucine; r = 0.998, S.E.cv = 0.15.
4. Conclusions
Our regression models, Equations (6) and (7), clearly show that by using the connectivity index 3χv it is possible to predict stability constants with an error 0.03–0.13 and 0.00–0.29 for log K1 and log β2, respectively, i.e. virtually within the limits of experimental error. Besides, maximal range of experimental values, 0.28 for log K1 of Co(Glycine) and 0.38 for log β2 of Co(Alanine)2, speaks strongly in favor of our model. All this leads to the conclusion that models for the prediction of stability constants based on connectivity index 3χv could provide a chemist the simple and efficient tool for planning his experiments and discussing his results.
Acknowledgment
This work was supported by Croatian Ministry of Science, Technology, Education and Sport (project 022-1770495-2901). We thank Dr Bono Lučić for helping us with E-Dragon software.
References
- 1.Irving H., Williams R.J.P. Order of stability of metal complexes. Nature. 1948;162:746–747. doi: 10.1038/162746a0. [DOI] [Google Scholar]
- 2.Irving H., Williams R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953:3192–3210. [Google Scholar]
- 3.Cannon R.D. Stabilities of chromium(II) complexes. J. Inorg. Nucl. Chem. 1976;38:1222–1223. doi: 10.1016/0022-1902(76)80257-6. [DOI] [Google Scholar]
- 4.Vinokurov E.G., Bondar V.V. Prediction of stability constants for Cr(III) and Cr(II) complexes. Russ. J. Coord. Chem. 2003;29:66–72. doi: 10.1023/A:1021851219309. [DOI] [Google Scholar]
- 5.Miličević A., Raos N. Prediction of stability constants. In: Morrison D.A. Ed., editor. Handbook of Inorganic Chemistry Research. Nova Science Publishers, Inc.; New York, NY, USA: 2010. pp. 267–294. [Google Scholar]
- 6.Raos N., Miličević A. Estimation of stability constants of coordination compounds using models based on topological indices. Arh. Hig. Rada Toksikol. 2009;60:123–128. doi: 10.2478/10004-1254-60-2009-1923. [DOI] [PubMed] [Google Scholar]
- 7.Miličević A., Raos N. Estimation of stability constants of copper(II) and nickel(II) chelates with dipeptides by using topological indices. Polyhedron. 2008;27:887–892. doi: 10.1016/j.poly.2007.11.017. [DOI] [Google Scholar]
- 8.Miličević A., Raos N. Prediction of stability of copper(II) and nickel(II) complexes with fructose-amino acids from the molecular-graph models developed on amino-acid chelates. Croat. Chem. Acta. 2007;80:557–563. [Google Scholar]
- 9.Trinajstić N. Chemical Graph Theory. 2nd. CRC Press; Boca Raton, FL, USA: 1992. [Google Scholar]
- 10.Janežić D., Miličević A., Nikolić S., Trinajstić N. Graph-Theoretical Matrices in Chemistry. In: Gutman I., editor. Mathematical Chemistry Monographs. University of Kragujevac and Faculty of Science; Kragujevac, Serbia: 2007. No. 3. [Google Scholar]
- 11.Kier L.B., Hall L.H. Molecular Connectivity and Drug Design. Academic Press; New York, NY, USA: 1976. [Google Scholar]
- 12.Graovac A., Gutman I., Trinajstić N. Lecture Notes in Chemistry. Springer-Verlag; Berlin, Germany: 1977. Topological Approach to the Chemistry of Conjugated Molecules. [Google Scholar]
- 13.Seybold P.G., May M., Bengal U.A. Molecular structure-property relationships. J. Chem. Educ. 1987;64:575–581. doi: 10.1021/ed064p575. [DOI] [Google Scholar]
- 14.Miličević A., Nikolić S., Trinajstić N. Toxicity of aliphatic ethers: A comparative study. Mol. Divers. 2006;10:95–99. doi: 10.1007/s11030-005-9006-0. [DOI] [PubMed] [Google Scholar]
- 15.Grgas B., Nikolić S., Paulić N., Raos N. Estimation of stability constants of copper(II) chelates with N-alkylated amino acids using topological indices. Croat. Chem. Acta. 1999;72:885–895. [Google Scholar]
- 16.Raos N., Branica G., Miličević A. The use of graph-theoretical models to evaluate two electroanalytical methods for determination of stability constants. Croat. Chem. Acta. 2008;81:511–517. [Google Scholar]
- 17.Miličević A., Raos N. Estimation of stability of coordination compounds by using topological indices. Polyhedron. 2006;25:2800–2808. doi: 10.1016/j.poly.2006.04.012. [DOI] [Google Scholar]
- 18.Miličević A., Raos N. Estimation of stability constants of mixed copper(II) chelates using valence connectivity index of the 3rd order derived from two molecular graph representations. Acta Chim. Slov. 2009;56:373–378. [Google Scholar]
- 19.Miličević A., Raos N. Estimation of stability constants of copper(II) chelates with triamines and their mixed complexes with amino acids by using topological indices and the overlapping spheres method. Polyhedron. 2007;26:3350–3356. doi: 10.1016/j.poly.2007.03.050. [DOI] [Google Scholar]
- 20.Miličević A., Raos N. Estimation of stability constants with connectivity index: Development of bivariate and multivariate linear models for copper(II) chelates with oligopeptides. Croat. Chem. Acta. 2009;82:633–639. [Google Scholar]
- 21.Miličević A., Raos N. Estimation of stability constants of cadmium(II) bis-complexes with amino acids by model based on 3χv connectivity index. Acta Chim. Slov. 2010;57:866–871. [PubMed] [Google Scholar]
- 22.Tetko I.V., Gasteiger J., Todeschini R., Mauri A., Livingstone D., Ertl P., Palyulin V.A., Radchenko E.V., Zefirov N.S., Makarenko A.S., Tanchuk V.Y., Prokopenko V.V. Virtual computational chemistry laboratory: design and description. J. Comput-Aided Mol. Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 23.VCCLAB, Virtual Computational Chemistry Laboratory. [(accessed on 1 September 2010)]. Available online: http://www.vcclab.org.
- 24.Todeschini R., Consonni V. Handbook of Molecular Descriptors. In: Mannhold R., Kubinyi H., Timmerman H., editors. Methods and Principles in Medicinal Chemistry. Vol. 11 Wiley-VCH; Weinheim, Germany: 2000. [Google Scholar]
- 25.Todeschini R., Consonni V. Molecular Descriptors for Chemoinformatics. In: Mannhold R., Kubinyi H., Folkers G., editors. Methods and Principles in Medicinal Chemistry. 2nd. Vol. 41 Wiley-VCH; Weinheim, Germany: 2009. [Google Scholar]
- 26. [(Accessed on 1 September 2010)]. Available online: http://cactus.nci.nih.gov/services/translate/
- 27.Kier L.B., Hall L.H. Molecular connectivity. J. Pharm. Sci. 1976;65:1806–1809. doi: 10.1002/jps.2600651228. [DOI] [PubMed] [Google Scholar]
- 28.Kier L.B., Hall L.H. Molecular Connectivity in Structure-Activity Analysis. Wiley; New York, NY, USA: 1986. [Google Scholar]
- 29.Randić M. On history of the Randić index and emerging hostility toward chemical graph theory. MATCH Commun. Math. Comput. Chem. 2008;59:5–124. [Google Scholar]
- 30.Miličević A., Raos N. Influence of chelate ring interactions on copper(II) chelate stability studied by connectivity index functions. J. Phys. Chem. A. 2008;112:7745–7749. doi: 10.1021/jp802018m. [DOI] [PubMed] [Google Scholar]
- 31.Lučić B., Trinajstić N. Multivariate regression outperforms several robust architectures of neural networks in QSAR modelling. J. Chem. Inf. Comput. Sci. 1999;39:121–132. doi: 10.1021/ci980090f. [DOI] [Google Scholar]
- 32.Maley L., Mellor D. Metal derivatives of 8-hydroxyquinoline 5-sulphonic acid and series of monocarboxylic mono-α-amino acids including histidine. Australian J. Sci. Res. A. 1949;92:579–594. [Google Scholar]
- 33.Monk C. Electolytes in solution of amino acids. Trans. Faraday Soc. 1951;47:285-291, 297-302. doi: 10.1039/tf9514700285. [DOI] [Google Scholar]
- 34.Anderson K.P., Greenhalgh W.O, Izatt R.M. Formation constants and enthalpy and entropy values for the association of H+ and Cu2+ with glycinate and phenylalanate ions in aqueous solution at 10, 25, and 40°. Inorg. Chem. 1966;5:2106–2109. doi: 10.1021/ic50046a005. [DOI] [Google Scholar]
- 35.Izatt R.M., Johnson H.D., Christensen J.J. Log Ki, ΔH°i, and ΔS°i values for the interaction of glycinate ion with H+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+ at 10, 25, and 40°. J. Chem. Soc. Dalton Trans. 1972:1152–1157. [Google Scholar]
- 36.Albert A. Quantitative studies of the avidity of naturally occurring substances for trace metals. Biochem. J. 1953;54:646–650. doi: 10.1042/bj0540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilands J.B. Metal and hydrogen-ion binding properties of cycloserine. Arch. Biochem. Biophys. 1956;62:151–162. doi: 10.1016/0003-9861(56)90098-4. [DOI] [PubMed] [Google Scholar]
- 38.Anderson K.P., Newel D.A., Izatt R.M. Formation constant, enthalpy, and entropy values for the association of alanine with H+ and Cu2+ at 10, 25, and 40°. Inorg. Chem. 1966;5:62–65. doi: 10.1021/ic50035a015. [DOI] [Google Scholar]
- 39.Albert A. Quantitative studies on the avidity of naturally occurring substances for trace metals. J. Biochem. 1950;47:531–540. doi: 10.1042/bj0470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta S., Leberman R., Rabin B. The chelation of metal ions by dipeptides and related substances. Trans. Faraday Soc. 1959;55:1982-1987, 2141-2151. doi: 10.1039/tf9595501982. [DOI] [Google Scholar]