Abstract
Background
Crosslinked polyethylene (XLPE) liners used for primary THA have demonstrated lower wear rates than noncrosslinked, conventional polyethylene (CPE) liners through the first decade of clinical service. However, little high-quality evidence is currently available regarding the second decade performance of these implants and it remains uncertain whether the onset of osteolysis has simply been delayed or if the wear associated with XLPE liners will remain low enough that osteolysis will not occur. It is also unknown how the potential reductions in wear and osteolysis will influence long-term revision rates.
Questions/purposes
Do patients who underwent THA with XLPE liners demonstrate (1) a lower rate of revision for wear-related complications; (2) a reduced wear rate; and (3) a lower frequency of osteolysis compared with those with CPE liners?
Methods
Over an 18-month period from 1999 to 2000, 226 patients who had 236 primary THAs consented to participate in a randomized controlled trial conducted at one institution. To be eligible for intraoperative randomization, patients had to be implanted with a 28-mm cobalt-chrome alloy femoral head, a 4-mm lateralized liner, and the same cup and stem design. Six patients with six THAs were excluded intraoperatively because they did not receive study components for reasons unrelated to the liner material. The remaining 230 THAs among 220 patients were randomized to XLPE liners or CPE liners. The mean age at surgery was 62 ± 11 years and there were no differences in age, gender, or body mass index among the groups. There was no differential loss to followup between the study groups; among patients not known to be deceased or having undergone revision, minimum 14-year radiographic followup is available for 85 THAs including 46 with XLPE and 39 with CPE liners. Polyethylene wear was measured radiographically using Martell’s Hip Analysis Suite and areas of osteolysis were evaluated before revision or at most recent followup. Revision rates at 15 years using reoperation for any reason and revision for wear or osteolysis were calculated using cumulative incidence considering patient death as a competing risk.
Results
The cumulative incidence of revision at 15 years using reoperation for wear-related complications as an endpoint was lower in the XLPE group than the CPE group (0%, 95% confidence interval [CI], 0%-0% versus 12%, 95% CI, 7%-19%; p < 0.001). Among unrevised THAs with minimum 14-year radiographic followup, the mean steady-state linear wear rate for THAs with XLPE liners was lower than the mean linear wear rate for the THAs with CPE liners (0.03 ± 0.05 versus 0.17 ± 0.09 mm/year; mean difference, 0.14; 95% CI, 0.11-0.17; p < 0.001). Osteolysis of any size was noted among 9% (four of 46) of the hips in the XLPE group and 46% (18 of 39) of the hips in the CPE group (odds ratio, 0.19; 95% CI, 0.07-0.51; p < 0.001).
Conclusions
This randomized study with followup into the second decade demonstrated reductions in revision, wear, and osteolysis associated with the use of XLPE. The low wear rates and absence of any mechanical failures among the XLPE liners at long-term followup affirm the durability of these components that did not incorporate antioxidants. Although osteolysis has not been eliminated, it occurs infrequently and has not caused any clinical problems to date.
Level of Evidence
Level I, therapeutic study.
Introduction
Highly crosslinked polyethylene (XLPE) liners used for primary THA have demonstrated substantially lower wear rates than conventional polyethylene (CPE) liners through the first decade of clinical service [6, 16, 40]. Because crosslinking is accompanied by a reduction in the ultimate tensile strength, fatigue strength, and elongation to failure of XLPE [5, 34, 35], characterizing the long-term clinical performance of XLPE is important to address concerns regarding the potential for liner fracture, in vivo polyethylene oxidation, and accelerated wear at long-term followup [13, 18, 23, 36]. Additionally, some investigators have questioned the bioreactivity of XLPE debris particles [20, 26, 27]. It also remains uncertain whether the wear associated with XLPE liners is below a threshold that would ever result in osteolysis or if the onset of osteolysis has simply been delayed. As a consequence, continued surveillance of XLPE liners is essential to document the long-term performance of these components.
In 1999, our institution initiated a randomized, institutional review board-approved study to compare wear rates, the frequency of osteolysis, and the risk of revision among patients undergoing primary THA who were randomized to either XLPE or CPE liners. At 5-year followup, the wear rates for the XLPE liners were substantially lower than those observed with the CPE liners [17]. Several other institutions have also reported very low wear rates with XLPE based on early data [4, 11, 12, 22, 25, 40]. Despite the substantial reduction in wear that was observed at 5-year followup, the patients’ clinical and functional outcomes did not differ between the XLPE and CPE groups [17]. At an average followup interval of 10 years, we found an 82% reduction in linear wear rates (0.04 versus 0.22 mm/year), a lower incidence of osteolysis, and improved survivorship among the XLPE liners [16]. Because the XLPE liners used in our study did not incorporate antioxidants, there are concerns that the wear performance of these components and other first-generation crosslinked liners may deteriorate over time as a result of in vivo oxidation or cyclic loading [10, 32, 37]. Because these types of components have been implanted in hundreds of thousands of patients undergoing THA, evaluating the clinical performance of XLPE liners at 15-year followup in the context of a prospective, randomized study is the best way to address contemporary concerns and provide data so clinicians and their patients can make evidence-based decisions.
The purpose of this study is to compare XLPE and CPE liners at 15-year followup with respect to three questions. Do patients who underwent THA with XLPE liners demonstrate (1) a lower rate of revision for wear-related complications; (2) a reduced wear rate; and (3) a lower frequency of osteolysis?
Patients and Methods
Beginning in January 1999 and continuing over an 18-month period, patients at our institution were enrolled in a prospective, randomized, institutional review board-approved study to compare wear rates, the frequency of osteolysis, and the risk of revision after THA using XLPE or CPE liners. The XLPE liners (MarathonTM; DePuy Orthopaedics, Warsaw, IN, USA) used for this study were treated with 5 Mrad (50 kGy) of gamma irradiation to induce crosslinking and subsequently heated above the melting temperature (150° C) to eliminate free radicals. This manufacturing process was designed to improve the polyethylene’s resistance to wear through increased crosslinking and the elimination of free radicals that render it susceptible to oxidative degradation while maintaining other physical properties such as ultimate strength and elongation to failure above regulated minimums [34]. The CPE liners (EnduronTM; DePuy Orthopaedics) were manufactured from the same polyethylene resin as XLPE liners but never irradiated. Both types of liners were machined and terminally sterilized with gas plasma, a noncrosslinking chemical surface treatment. Based on the manufacturing methods, the XLPE and CPE liners would not have had free radicals at the time of implantation and neither liner material incorporated antioxidants. Unless they were excluded intraoperatively, all patients who consented to participate in the study were implanted on the acetabular side with a press-fit porous-coated hemispherical cup (Duraloc® 100; DePuy Orthopaedics) without supplemental screw fixation and a 4-mm lateralized liner. The cup featured a single apical dome hole used for implantation that was filled with a threaded plug after the cup was impacted. On the femoral side, an extensively porous-coated stem (Anatomic Medullary Locking [AML®], Solution®, or Prodigy®; DePuy Orthopaedics) was used with a modular 28-mm cobalt-chrome alloy femoral head.
All patients undergoing primary THA were eligible to participate in this study. A total of 226 patients (236 hips) consented to participate. Six patients (six hips) were excluded from the study intraoperatively and not randomized because intraoperative considerations unrelated to the liner material led to the use of components that were different than those specified in the protocol. Of these, three patients received 32-mm femoral heads to improve intraoperative stability. To address issues of leg length and hip stability, one patient received a deep-profile cup, whereas another patient was implanted with a neutral liner without offset instead of a 4-mm lateralized liner. One additional patient with soft osteoporotic bone was implanted with a cup featuring multiple cavitary holes to afford the option for supplemental screw fixation. The remaining 230 THAs (220 patients) comprise the population for this study. At the time of surgery, these patients were assigned to receive XLPE or CPE liners according to a randomization sequence generated by an independent statistician (MJS). The randomization scheme was designed to have equal sample sizes after every 50 patients. At the time of surgery, only the patient was blinded to the type of liner they received. However, when radiographic analyses of wear and osteolysis were performed, the analysts were blinded to the type of liner.
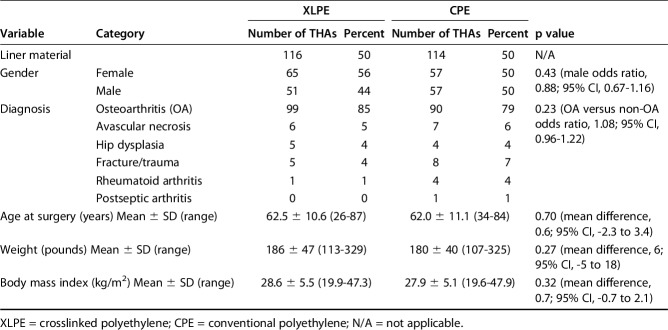
The mean age at surgery of the patients receiving XLPE liners was 63 ± 11 years compared with 62 ± 11 years for the patients receiving CPE liners (mean difference, 0.6; 95% confidence interval [CI], -2.3 to 3.4; p = 0.70). There were no differences in gender, preoperative diagnosis, weight, or body mass index among the groups (Table 1). Among the 10 patients with bilateral hip replacements, two had both hips randomized to XLPE, three had both hips randomized to CPE, and five had one hip randomized to XLPE and the other randomized to CPE.
Table 1.
Demographics
Patients enrolled in this study were asked to return for routine postoperative followup annually for the first 2 years after surgery and every 2 to 3 years thereafter. At followup, patients were asked to complete an unsupervised questionnaire, were examined by an orthopaedic surgeon, and had standardized radiographs taken including AP pelvic and lateral views. Patients who could not return to our institution were asked to send in radiographs, complete a patient questionnaire, and respond to questions about reoperations and complications.
A radiographic review was conducted among all unrevised THAs with minimum 14-year followup radiographs. In addition, prerevision radiographs were analyzed for patients who had reoperations involving removal of the primary liner. Osteolysis was assessed by a single experienced surgeon (CAE) who was blinded to the type of polyethylene liner. Femoral and acetabular osteolytic lesions were identified by comparing the most recent followup radiographs with perioperative views. Osteolysis was defined as an area of localized trabecular bone loss or cortical erosion that was not apparent on the preoperative or immediate postoperative radiograph. To obtain lesion sizes, the defects were outlined on the AP pelvic and lateral radiographs. The area of each lesion was measured using Martell's Hip Analysis Suite software (version 4.5; University of Chicago, Chicago, IL, USA). If a lesion grew from a preexisting cyst that could be identified on an immediate or early postoperative radiograph, the osteolytic area was computed as the total defect area at most recent followup minus the initial cyst area. The area of osteolysis for the pelvis and femur was defined as the maximum area measured on either the AP pelvic or lateral radiograph. The total osteolysis for each hip was computed by adding the maximum pelvic and femoral osteolysis areas. Hips with a total area of at least 1.5 cm2 were considered to have clinically important osteolysis [16].
A single reviewer (HH), who was also blinded to the type of polyethylene liner, evaluated femoral head penetration using serial AP pelvic radiographs. Two-dimensional head penetration was determined for each followup radiograph relative to the immediate postoperative (nominal 6-week followup) reference view using Martell’s Hip Analysis Suite version 8.0 with elliptical correction, a validated, computer-assisted technique [33]. Based on the total head penetration calculated using the first postoperative radiograph and most recent radiograph, a head penetration rate was calculated for each THA by dividing the total head penetration by the duration of followup between the radiographs. Linear wear rates were also calculated for all THAs that had a minimum of three followup radiographs with the first radiograph taken at least 0.75 years after surgery to exclude early head penetration associated with liner deformation/creep. To do this, a least-squares linear regression based on the magnitude of the wear vector versus time in situ was used to calculate the slope of a best-fit line [42]. The slope from this regression represented the steady-state linear wear rate. We also report the number of THAs with linear wear rates above 0.10 mm/year, which has been proposed as an osteolysis threshold [14].
Statistical Analysis
Cumulative incidence considering patient death as a competing risk was used to compute revision rates using reoperation for any reason and revision (including ball and liner exchanges) for wear and/or osteolysis and is reported at 15-year followup ± 95% CI. Differences in cumulative incidence rates were evaluated using Gray’s test. Comparisons of continuous variables between groups were performed using an independent-samples t-test. Comparisons of binary categorical data were evaluated using Fisher's exact test. A p value of 0.05 was used as the threshold for statistical significance. All statistical analyses were performed with SPSS (Chicago, IL, USA) with the exception of cumulative incidence that was evaluated using SAS Studio (Cary, NC, USA). A power analysis was used to determine the number of THAs to be enrolled. Based on institutional wear data, the linear wear rate for the CPE liners was assumed to be 0.08 ± 0.05 mm/year. Assuming a mean wear rate difference of 0.015 mm/year between the groups and a SD of 0.05 mm/year for both groups, it was determined that 352 THAs would be required for a power of 80% based on a two-tailed independent-samples t-test with a criterion for significance (α) equal to 0.05. To compensate for patients lost to followup, the original enrollment goal was 400 patients. However, enrollment was ended after 236 patients had consented to participate owing to a change in the process used to manufacture the liners. Because the type of liner was assigned at the time of surgery, it was impossible for patients to cross over from one group to another. As a consequence, we used an “intention-to-treat” analysis, but the “as-treated” analysis would be the same.
The outcome of a THA was considered to be known if the liner had been revised, the patient was known to be deceased, or minimum 14-year followup data were available. The followup data could include radiographs or basic information regarding whether the THA had been revised. THAs without known outcomes were considered to have incomplete followup. For these THAs, the most recent followup interval was used for cumulative incidence calculations. Among the 116 THAs (114 patients) randomized to XLPE, 85% (99 THAs in 97 patients) have known outcomes with a mean followup of 14.2 ± 4.3 years. This includes three THAs among three patients with liner revisions at a mean followup of 9.6 ± 2.3 years, 32 unrevised THAs among 32 patients who died at a mean followup of 10.1 ± 5.2 years, 46 THAs among 44 patients with minimum 14-year radiographs who have a mean followup of 16.3 ± 0.7 years, and 18 THAs among 18 patients known to remain in service at least 14 years after surgery with a mean followup of 16.6 ± 0.6 years (Fig. 1). The 17 XLPE hips among 17 patients with incomplete followup have a mean followup of 10.0 ± 2.3 years. Among the 114 THAs (111 patients) randomized to CPE, 86% (98 THAs in 96 patients) have known outcomes with a mean followup of 13.7 ± 3.9 years. This includes 19 THAs among 19 patients with liner revisions at a mean followup of 11.7 ± 4.0 years, 33 unrevised THAs among 33 patients who died at a mean followup of 11.0 ± 3.8 years, 39 THAs among 37 patients with minimum 14-year radiographs that have a mean followup of 16.4 ± 0.7 years, and seven THAs among seven patients known to remain in service at least 14 years after surgery with a mean followup of 16.6 ± 0.7 years. The 16 CPE hips among 15 patients with incomplete followup have a mean followup of 8.6 ± 3.0 years.
Fig. 1.
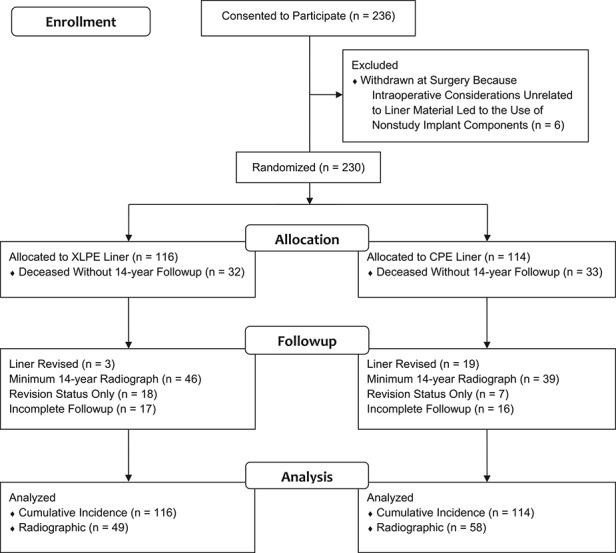
The CONSORT flow diagram illustrates the status of the 236 THAs among patients who consented to participate in this study.
Results
The cumulative incidence of revision at 15 years using reoperation for wear-related complications as an endpoint was lower in the XLPE group than the CPE group (0%, 95% CI, 0%-0% versus 12%, 95% CI, 7%-19%; p < 0.001). Using reoperation for any reason as an endpoint, the cumulative incidence of revision at 15 years was also lower in the XLPE group than the CPE group (4%, 95% CI, 1%-8% versus 14%, 95% CI, 8%-21%; p = 0.001). Among the 116 THAs randomized to XLPE liners, there have been four reoperations including one open reduction without component exchange at 4.3 years after the index procedure and three liner and head exchanges for recurrent dislocation at a mean followup of 9.6 ± 2.3 years. None of these four hips had osteolysis before reoperation. Among the 114 THAs randomized to CPE liners, there have been 19 liner and ball exchanges including two for recurrent dislocation at 3.8- and 9.6-year followup and 17 related to wear at a mean followup of 12.3 ± 3.7 years. The mean linear wear rate for these 17 THAs was 0.43 ± 0.15 mm/year and the mean size of the osteolytic lesions on the prerevision radiograph was 5.4 ± 3.7 cm2 . Seven of the 17 hips also had an osteolytic fracture including five involving the greater trochanter, one involving the lesser trochanter, and one involving the pubic ramus and greater trochanter. None of these fractures were treated with fixation at the time of revision, but larger osteolytic lesions were typically grafted. Despite the size of the osteolytic lesions and the presence of fractures in the CPE group, all cups and stems were found to be well fixed at the time of revision, none of the cups or stems have been revised, and no reoperations for loosening or infection have occurred in either group.
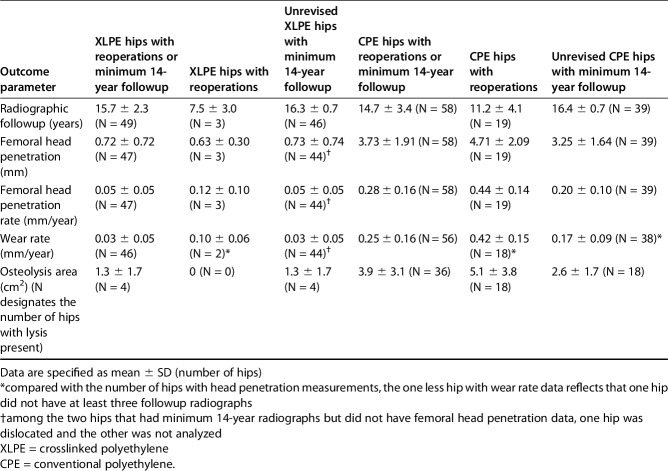
The femoral head penetration rate was lower in the XLPE group than the CPE group among unrevised hips followed for at least 14 years (Table 2; 0.05 ± 0.05 versus 0.20 ± 0.10; mean difference, 0.15; 95% CI, 0.12-0.19 mm/year; p < 0.001). Among these THAs, 9% (four of 44) of XLPE and 85% (33 of 39) of CPE THAs had penetration rates > 0.10 mm/year. For the THAs with CPE liners, the 17 hips that underwent wear-related revisions had higher head penetration rates compared with the 39 with minimum 14-year radiographic followup that were not revised (0.44 ± 0.15 versus 0.20 ± 0.10; mean difference, 0.24; 95% CI, 0.16-0.33 mm/year; p < 0.001). Among unrevised THAs with minimum 14-year radiographic followup and at least three serial head penetration measurements, the steady-state linear wear rates for the XLPE liners were lower than the linear wear rates for the CPE liners (0.03 ± 0.05 versus 0.17 ± 0.09; mean difference, 0.14; 95% CI, 0.11-0.18 mm/year; p < 0.001). The steady-state linear wear rate was > 0.10 mm/year for 7% (three of 44) of the XLPE and 76% (29 of 38) of the CPE THAs. For all hips with at least three serial head penetration measurements that had reoperations or minimum 14-year radiographic followup (Fig. 2), the THAs with XLPE liners had lower linear wear rates than the THAs with CPE liners (Table 2; 0.03 ± 0.05 versus 0.25 ± 0.16; mean difference, 0.22; 95% CI, 0.17-0.27 mm/year; p < 0.001). Among these THAs, the steady-state linear wear rate was > 0.10 mm/year for 9% (four of 46) of those with XLPE liners and 84% (47 of 56) of those with CPE liners.
Table 2.
Outcome among XLPE and CPE hips
Fig. 2.
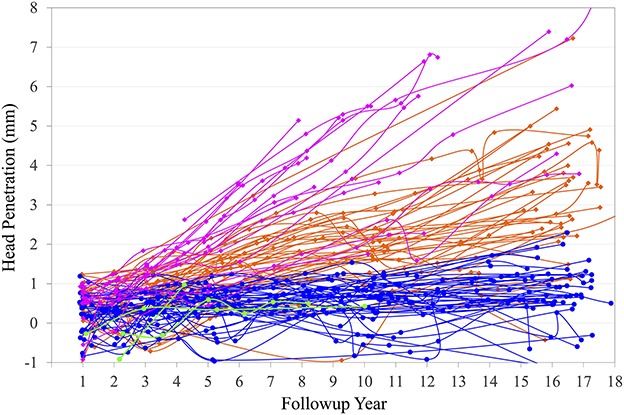
Serial head penetration plots for THAs with XLPE liners including those that had reoperations (green) and those that did not (blue) versus the CPE liners that had reoperations (magenta) and those that did not (orange) illustrate the reduced wear associated with the XLPE liners. The THAs with CPE liners that underwent reoperations tended to have the highest wear rates. Penetration measurements for individual THAs are connected by continuous lines.
Based on the radiographic review of unrevised THAs with minimum 14-year radiographic followup, osteolysis of any size was less common in the XLPE group than the CPE group (Table 2; 9% [four of 46] versus 46% [18 of 39]; odds ratio, 0.19; 95% CI, 0.07-0.51; p < 0.001). The incidence of osteolysis with an area of at least 1.5 cm2 was also lower for the XLPE group (2% [one of 46] versus 31% [12 of 39]; odds ratio, 0.07; 95% CI, 0.01-0.52; p < 0.001). The single hip in the XLPE group that had clinically important osteolysis demonstrated a pelvic defect behind the dome hole measuring 2.9 cm2 on the lateral view and femoral defects on AP and lateral views (Fig. 3). For this hip, the area of the femoral osteolysis on the AP view was 0.9 cm2 in Gruen Zone 1 and on the lateral view measured 0.7 cm2 in Gruen Zone 8 and 0.3 cm2 in Gruen Zone 14. Among the other three XLPE hips with osteolysis, one hip had osteolysis that was only apparent on the lateral view with a pelvic defect measuring 0.5 cm2 and a femoral lesion in Gruen Zone 8 measuring 0.1 cm2, another hip also had osteolysis that was only apparent on the lateral view with femoral lesions measuring 0.1 cm2 in Gruen Zones 8 and 14, and the remaining hip had a 0.5-cm2 pelvic osteolytic lesion behind the dome hole previously reported at 11-year followup [16] that grew from a preexisting cyst and did not progress between 11- and 16-year followup. Among the 39 unrevised hips with CPE liners that had at least 14-year radiographic followup, 18 had osteolysis with a mean area of 2.6 ± 1.7 cm2. Including the revised THAs and those with minimum 14-year radiographic followup, there was a lower incidence of fractures associated with osteolytic lesions among the hips with XLPE liners compared with the CPE group (0% [zero of 49] versus 16% [nine of 58], p = 0.004). Although there were no fractures associated with osteolysis in the XLPE group, one female patient sustained a nondisplaced greater trochanter fracture in conjunction with osteoporosis 15.1 years after surgery at the age of 80 years. In addition to the seven fractures previously noted among revised patients with CPE liners, there were three additional greater trochanter fractures among patients who have not been revised to date. These included two fractures associated with osteolysis and one fracture without osteolysis diagnosed 14.5 years after surgery in an 85-year-old female patient. Including all fractures among patients who were revised or had at least 14-year followup, there was a lower incidence of postoperative fractures among the XLPE group compared with the CPE group (2% [one of 49] versus 17% [10 of 58]; odds ratio, 0.12; 95% CI, 0.02-0.89; p = 0.01).
Fig. 3.
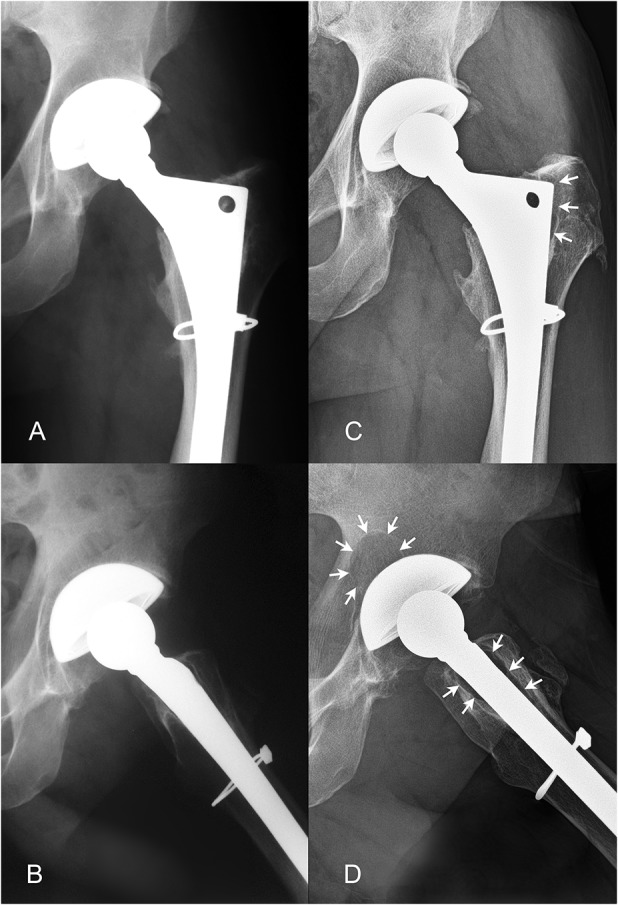
The patient with XLPE who had osteolysis exceeding 1.5 cm2 in the current study demonstrated no evidence of osteolysis at 8-year followup on AP (A) or lateral (B) radiographs. At 16-year followup, proximal femoral osteolysis (designated by the white arrows) was noted on the AP (C) and lateral radiographs (D). An acetabular lesion behind the dome hole with an area of 2.9 cm2 was also noted on the lateral view (D). The patient was a man who was 43 years of age at the time of his primary THA and who had a head penetration rate of 0.07 mm/year and a linear wear rate of 0.05 mm/year.
Discussion
Polyethylene wear can be influenced by many factors. To evaluate the differences between XLPE and CPE, we used a prospective, randomized study design with the same cup design, polyethylene liner geometry, femoral head diameter, head material, and stem type to control for potential confounding factors. At 15-year followup, we found a lower cumulative incidence of revision, markedly reduced wear rates, and a substantially decreased incidence of osteolysis among THAs with XLPE liners compared with those with CPE liners.
The main weakness of the current study is the incomplete followup. Thirty-three (14%) of the original 230 hips among patients not known to be deceased have less than 14-year followup. Radiographic followup was particularly difficult to obtain. Excluding the 65 THAs among patients who are known to be dead and the 22 THAs that underwent revisions involving a liner exchange yields a minimum 14-year radiographic followup rate of 59% (85 of 143). Although current followup was not available for all patients, the rates of followup in the XLPE and CPE groups were similar. Despite the fact that we did not meet our enrollment goal and some patients had incomplete followup, the number of hips available for the current study was adequate to demonstrate a difference in the cumulative incidence of revision, wear, and osteolysis. Because the 33 THAs classified as having incomplete followup actually had a mean followup of 9.3 ± 2.7 years, the CIs for the cumulative incidence of revision remained less than ± 10% at 15-year followup. Although the statistical methodology for this study was predicated on independent samples, 10 of the 220 patients had bilateral hip replacements. While prior studies have shown that bilateral THAs do not have identical wear rates [28, 38], they are also not truly independent. However, the impact of these 20 THAs on the outcome of the study was likely minimal because nine hips were randomized to XLPE and 11 to CPE. Other limitations of the study include the absence of validated patient-reported outcome scores. Although the components of the Harris hip score were collected from a patient questionnaire and physical evaluation, all of the components were not always present and the date of the most recent followup questionnaire did not always correspond to the most recent examination date, particularly when patients could not return to our institution for followup. However, with the available data, there was no evidence of differences in Harris hip score preoperatively or at followup among the XLPE and CPE groups and the improvement in Harris hip scores from the preoperative to most recent followup interval for both groups was similar (data not shown). It should also be noted that radiographs instead of three-dimensional techniques such as MRI or CT were used to evaluate osteolysis. Although larger osteolytic lesions are often apparent on an AP radiograph [9, 29, 41], we reviewed both AP and lateral radiographs for this study to facilitate the identification of all defects. Additionally, defects identified on followup radiographs were compared with perioperative views to exclude preexisting osteoarthritic cysts from our interpretation of osteolysis.
This study reaffirms the difference in revision rates that we identified at 10-year followup [16]. Using reoperation for any reason as an endpoint, the 15-year cumulative incidence of revision is 4% for XLPE compared with 14% for CPE liners. We are not aware of another prospective, randomized study that has shown a decreased revision rate comparing XLPE with CPE. However, registry data have shown lower revision rates with XLPE [1, 39]. Using data derived from the Kaiser Permanente Total Joint Replacement Registry for 26,823 primary THAs performed between April 2001 and December 2011, Paxton et al. [39] found that metal-on-CPE THA bearing surfaces have a higher risk of revision compared with metal-on-XLPE bearing surfaces. In that study, the cumulative incidence of revision at 7-year followup was 5.4% (95% CI, 4.4%-6.7%) for metal-on-CPE and 2.8% (95% CI, 2.6%-3.2%) for metal-on-XLPE. Comparing this study with ours, we observed a larger difference in revision rates at longer followup.
We have reaffirmed the wear data we presented in our 5- and 10-year reports on this cohort of patients. In the current study, the mean steady-state linear wear rate at minimum 14-year followup for unrevised XLPE liners was 83% lower than the linear wear rates for CPE liners (0.03 versus 0.17 mm/year). Importantly, because the current linear wear rates have not increased compared with our previously reported rate of 0.04 mm/year at 10-year followup, we found no evidence of increased wear in the second decade of clinical service, which is consistent with the findings of other investigators [24]. We also believe our results are applicable to other XLPEs. A systematic analysis of XLPE and CPE [30] reported wear rates for liners from several different manufacturers that are quite similar to our linear wear rates. In that analysis, the mean weighted average two-dimensional penetration rate for XLPE was 0.042 mm/year and 0.137 mm/year for CPE. In addition to the systematic review, the mean linear wear rate of our XLPE liners is in the same range as three more recent studies of XLPE that have reported wear rates of 0.003 to 0.04 mm/year at 10 years [3, 21, 24].
Based on the radiographic review of THAs with minimum 14-year followup, the incidence of any osteolysis was much lower in THAs with XLPE liners than in those with CPE liners. Despite the presence of osteolysis among many of the THAs with CPE liners, none of the cups or stems among any of the THAs in this study has loosened. Although component loosening is often cited as a consequence of osteolysis, it does not appear to be a problem with the type of porous-coated components used for this study. Instead, the most common complications associated with osteolysis were trochanteric fractures. This finding is consistent with a study by Claus et al. [9] that reported a 4.3% incidence of trochanteric fractures resulting from osteolysis at a mean followup of 12.2 years among 208 THAs that had gamma-sterilized CPE liners with extensively porous-coated (AML) stems. Among the four THAs with XLPE liners that demonstrated evidence of osteolysis in our current report, three hips had lesion areas that were < 1.5 cm2. These three included one hip with pelvic osteolysis previously reported at 10-year followup that did not progress between 10- and 15-year followup. Only one hip in the XLPE group had osteolysis exceeding 1.5 cm2. Because the 2.9-cm2 pelvic lesion for this hip was located behind the dome hole, we cannot preclude the possibility that fluid pressure may have contributed to the development of this defect [43, 44]. The low incidence of osteolysis among THAs with XLPE liners is consistent with the report by Lachiewicz et al. [31] that found small (< 1 cm) osteolytic lesions among 14% of hips (12 of 84) at a mean followup of 11 years (range, 10-14 years) and Babovic and Trousdale [2] who found no radiographic evidence of osteolysis or component loosening among 54 THAs at minimum 10-year followup among patients who were < 50 years of age at the time of surgery (mean age, 39 years). A systematic review by Kurtz et al. [30] found that XLPE liners had an 87% lower risk of osteolysis. Consistent with in vitro investigations [15, 19] and other clinical studies [7], the results from the current study do not support the notion that wear debris from XLPE has increased bioreactivity compared with CPE.
In conclusion, XLPE has substantially lower wear, a reduced incidence of osteolysis, and a decreased revision rate compared with CPE at 15-year followup. There have also been no mechanical failures or fractures among the XLPE liners. We observed a 9% (four of 46) incidence of osteolysis among the XLPE liners at 15-year followup, but only one (2%) THA demonstrated osteolysis with an area of at least 1.5 cm2. Although the incidence and size of osteolysis have been substantially reduced with XLPE liners, because it has not been completely eliminated, we currently recommend continued radiographic evaluation of active patients with XLPE every 5 years. This prospective, randomized study is one of the first investigations to show a long-term clinical benefit from use of XLPE that is becoming more apparent in the second decade of in vivo service. Although the wear performance is substantially improved and osteolysis is rare with XLPE, dislocation remains a concern with the use of 28-mm femoral heads. We would also caution that the results seen with 28-mm femoral heads are not necessarily applicable to larger diameter heads [8]. It is conceivable that a greater volume of debris generated by larger femoral head diameters compared with the 28-mm components used for this study could cause an increased incidence of osteolysis at 15 years.
Acknowledgments
We thank Michael J. Sheridan ScD, who generated the randomization sequence used for this study. The authors also gratefully acknowledge a gift from the estate of Jeanette L. Blankenship that supported this study.
Footnotes
The institution of the authors (RHH, HH, SS, ACW, CAE) has received, during the study period, funding from DePuy Synthes Joint Reconstruction, a Division of DePuy Orthopaedics, Inc (Warsaw, IN, USA), a Johnson & Johnson company (New Brunswick, NJ, USA), and funding from Inova Health System (Falls Church, VA, USA). One of the authors (CAE) lists the following relevant financial activities outside of this work and/or any other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: DePuy Orthopaedics in the amount of more than USD 1,000,001 and Smith & Nephew (Memphis, TN, USA) in the amount of USD 10,000 to USD 100,000. Funding for this study was provided by the Inova Health System, Jeanette L. Blankenship, and DePuy Synthes Joint Reconstruction, a Division of DePuy Orthopaedics, Inc, a Johnson & Johnson company.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Anderson Orthopaedic Research Institute, Alexandria, VA, USA.
References
- 1.Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. Adelaide, Australia: AOA; 2015. [Google Scholar]
- 2.Babovic N, Trousdale RT. Total hip arthroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;28:815–817. [DOI] [PubMed] [Google Scholar]
- 3.Bedard NA, Callaghan JJ, Stefl MD, Willman TJ, Liu SS, Goetz DD. Fixation and wear with a contemporary acetabular component and cross-linked polyethylene at minimum 10-year follow-up. J Arthroplasty. 2014;29:1961–1969. [DOI] [PubMed] [Google Scholar]
- 4.Bitsch RG, Loidolt T, Heisel C, Ball S, Schmalzried TP. Reduction of osteolysis with use of Marathon cross-linked polyethylene. A concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2008;90:1487–1491. [DOI] [PubMed] [Google Scholar]
- 5.Bradford L, Baker D, Ries MD, Pruitt LA. Fatigue crack propagation resistance of highly crosslinked polyethylene. Clin Orthop Relat Res. 2004;429:68–72. [DOI] [PubMed] [Google Scholar]
- 6.Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Multicenter Study Group, Malchau H. The 2012 John Charnley Award. Clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop Relat Res. 2013;471:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broomfield JA, Malak TT, Thomas GE, Palmer AJ, Taylor A, Glyn-Jones S. The relationship between polyethylene wear and peri-prosthetic osteolysis in total hip arthroplasty at 12 years in a randomized controlled trial cohort. J Arthroplasty. 2017;32:1186–1191. [DOI] [PubMed] [Google Scholar]
- 8.Burstein AH. The appropriate variable to compare wear in total hip prostheses: commentary on an article by Howie DW, et al. The wear rate of highly cross-linked polyethylene in total hip replacement is not increased by large articulations. A randomized controlled trial. J Bone Joint Surg Am. 2016;98:e98. [DOI] [PubMed] [Google Scholar]
- 9.Claus AM, Engh CA, Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA., Sr Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1519–1526. [DOI] [PubMed] [Google Scholar]
- 10.Currier BH, Van Citters DW, Currier JH, Collier JP. In vivo oxidation in remelted highly cross-linked retrievals. J Bone Joint Surg Am. 2010;92:2409–2418. [DOI] [PubMed] [Google Scholar]
- 11.Digas G, Kärrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78:746–754. [DOI] [PubMed] [Google Scholar]
- 12.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. [DOI] [PubMed] [Google Scholar]
- 13.Duffy GP, Wannomae KK, Rowell SL, Muratoglu OK. Fracture of a cross-linked polyethylene liner due to impingement. J Arthroplasty. 2009;24:158e15–19. [DOI] [PubMed] [Google Scholar]
- 14.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. [DOI] [PubMed] [Google Scholar]
- 15.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng H. 2002;216:111–122. [DOI] [PubMed] [Google Scholar]
- 16.Engh CA, Jr, Hopper RH, Huynh C, Ho H, Sritulanondha S, Engh CA. A prospective, randomized study of cross-linked and noncross-linked polyethylene for total hip arthroplasty at 10-year follow-up. J Arthroplasty. 2012;27(Suppl 1):2–7. [DOI] [PubMed] [Google Scholar]
- 17.Engh CA, Jr, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Jr, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked Marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21(Suppl 2):17–25. [DOI] [PubMed] [Google Scholar]
- 18.Galvin A, Kang L, Tipper J, Stone M, Ingham E, Jin Z, Fisher J. Wear of crosslinked polyethylene under different tribological conditions. J Mater Sci Mater Med. 2006;17:235–243. [DOI] [PubMed] [Google Scholar]
- 19.Galvin AL, Tipper JL, Ingham E, Fisher J. Nanometre size wear debris generated from crosslinked and non-crosslinked ultra high molecular weight polyethylene in artificial joints. Wear. 2005;259:977–983. [Google Scholar]
- 20.Galvin AL, Tipper JL, Jennings LM, Stone MH, Jin ZM, Ingham E, Fisher I. Wear and biological activity of highly crosslinked polyethylene in the hip under low serum protein concentrations. Proc Inst Mech Eng H. 2007;221:1–10. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A. New polyethylenes in total hip replacement. A ten- to 12-year followup study. Bone Joint J. 2013;95:326–322. [DOI] [PubMed] [Google Scholar]
- 22.Geerdink CH, Grimm B, Ramakrishnan R, Rondhuis J, Verburg AJ, Tonino AJ. Crosslinked polyethylene compared to conventional polyethylene in total hip replacement: pre-clinical evaluation, in-vitro testing and prospective clinical follow-up study. Acta Orthop. 2006;77:719–725. [DOI] [PubMed] [Google Scholar]
- 23.Gencur SJ, Rimnac CM, Kurtz SM. Fatigue crack propagation resistance of virgin and highly crosslinked, thermally treated ultra-high molecular weight polyethylene. Biomaterials. 2006;27:1550–1557. [DOI] [PubMed] [Google Scholar]
- 24.Glyn-Jones S, Thomas GE, Garfjeld-Roberts P, Gundle R, Taylor A, McLardy-Smith P, Murray DW. The John Charnley Award: Highly crosslinked polyethylene in total hip arthroplasty decreases long-term wear: a double-blind randomized trial. Clin Orthop Relat Res. 2015;473:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heisel C, Silva M, dela Rosa MA, Schmalzried TP. Short-term in vivo wear of cross-linked polyethylene. J Bone Joint Surg Am. 2004;86:748–751. [DOI] [PubMed] [Google Scholar]
- 26.Illgen RL, 2nd, Bauer LM, Hotujec BT, Kolpin SE, Bakhtiar A, Forsythe TM. Highly crosslinked vs conventional polyethylene particles: relative in vivo inflammatory response. J Arthroplasty. 2009;24:117–124. [DOI] [PubMed] [Google Scholar]
- 27.Illgen RL, 2nd, Forsythe TM, Pike JW, Laurent MP, Blanchard CR. Highly crosslinked vs conventional polyethylene particles–an in vitro comparison of biologic activities. J Arthroplasty. 2008;23:721–731. [DOI] [PubMed] [Google Scholar]
- 28.Joshi A, Ilchmann T, Markovic L. Socket wear in bilateral simultaneous total hip arthroplasty. J Arthroplasty. 2001;16:117–120. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura N, Pappedemos PC, Duffy PR, 3rd, Stepniewski AS, Hopper RH, Jr, Engh CA, Jr, Engh CA. The value of anteroposterior pelvic radiographs for evaluating pelvic osteolysis. Clin Orthop Relat Res. 2006;453:239–245. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachiewicz PF, Soileau ES, Martell JM. Wear and osteolysis of highly crosslinked polyethylene at 10 to 14 years: the effect of femoral head size. Clin Orthop Relat Res. 2016;474:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald D, Sakona A, Ianuzzi A, Rimnac CM, Kurtz SM. Do first-generation highly crosslinked polyethylenes oxidize in vivo? Clin Orthop Relat Res. 2011;469:2278–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with the use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. [DOI] [PubMed] [Google Scholar]
- 34.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157–167. [DOI] [PubMed] [Google Scholar]
- 35.Medel FJ, Peña P, Cegoñino J, Gómez-Barrena E, Puértolas JA. Comparative fatigue behavior and toughness of remelted and annealed highly crosslinked polyethylenes. J Biomed Mater Res B Appl Biomater. 2007;83:380–390. [DOI] [PubMed] [Google Scholar]
- 36.Minoda Y, Kobayashi A, Sakawa A, Aihara M, Tada K, Sugama R, Iwakiri K, Ohashi H, Takaoka K. Wear particle analysis of highly crosslinked polyethylene isolated from a failed total hip arthroplasty. J Biomed Mater Res B Appl Biomater. 2008;86:501–505. [DOI] [PubMed] [Google Scholar]
- 37.Miura Y, Hasegawa M, Sudo A, Pezzotti G, Puppulin L. In-vivo degradation of middle-term highly cross-linked and remelted polyethylene cups: modification induced by creep, wear and oxidation. J Mech Behav Biomed Mater. 2015;51:13–24. [DOI] [PubMed] [Google Scholar]
- 38.Orishimo KF, Sychterz CJ, Hopper RH, Jr, Engh CA. Can component and patient factors account for the variance in wear rates among bilateral total hip arthroplasty patients? J Arthroplasty. 2003;18:154–160. [DOI] [PubMed] [Google Scholar]
- 39.Paxton EW, Inacio MC, Namba RS, Love R, Kurtz SM. Metal-on-conventional polyethylene total hip arthroplasty bearing surfaces have a higher risk of revision than metal-on-highly crosslinked polyethylene: results from a US registry. Clin Orthop Relat Res. 2015;473:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Röhrl SM, Nivbrant B, Nilsson KG. No adverse effects of submelt-annealed highly crosslinked polyethylene in cemented cups: an RSA study of 8 patients 10 years after surgery. Acta Orthop. 2012;83:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shon WY, Gupta S, Biswal S, Han SH, Hong SJ, Moon JG. Pelvic osteolysis relationship to radiographs and polyethylene wear. J Arthroplasty. 2009;24:743–750. [DOI] [PubMed] [Google Scholar]
- 42.Sychterz CJ, Engh CA, Jr, Yang AM, Engh CA. Analysis of temporal wear patterns of porous-coated acetabular components: distinguishing between true wear and so-called bedding-in. J Bone Joint Surg Am. 1999;81:821–830. [DOI] [PubMed] [Google Scholar]
- 43.van der Vis H, Aspenberg P, de Kleine R, Tigchelaar W, van Noorden CJ. Short periods of oscillating fluid pressure directed at a titanium-bone interface in rabbits lead to bone lysis. Acta Orthop Scand. 1998;69:5–10. [DOI] [PubMed] [Google Scholar]
- 44.Walter WL, Walter WK, O'Sullivan M. The pumping of fluid in cementless cups with holes. J Arthroplasty. 2004;19:230–234. [DOI] [PubMed] [Google Scholar]