Abstract
Background
Previous studies have shown that venous thromboembolism (VTE) is a complication associated with neoplastic disease and major orthopaedic surgery. However, many potential risk factors remain undefined.
Questions/purposes
(1) What proportion of patients develop symptomatic VTE after surgery for long bone metastases? (2) What factors are associated with the development of symptomatic VTE among patients receiving surgery for long bone metastases? (3) Is there an association between the development of symptomatic VTE and 1-year survival among patients undergoing surgery for long bone metastases? (4) Does chemoprophylaxis increase the risk of wound complications among patients undergoing surgery for long bone metastases?
Methods
A retrospective study identified 682 patients undergoing surgical treatment of long bone metastases between 2002 and 2013 at the Massachusetts General Hospital and Brigham and Women's Hospital. We included patients 18 years of age or older who had a surgical procedure for impending or pathologic metastatic long bone fracture. We considered the humerus, radius, ulna, femur, tibia, and fibula as long bones; metastatic disease was defined as metastases from solid organs, multiple myeloma, or lymphoma. In general, we used 40 mg enoxaparin daily for lower extremity surgery and 325 mg aspirin daily for lower or upper extremity surgery. The primary outcome was a VTE defined as any symptomatic pulmonary embolism (PE) or symptomatic deep vein thrombosis (DVT; proximal and distal) within 90 days of surgery as determined by chart review. The tertiary outcome was defined as any documented wound complication that might be attributable to chemoprophylaxis within 90 days of surgery. At followup after 90 days and 1 year, respectively, 4% (25 of 682) and 8% (53 of 682) were lost to followup. Statistical analysis was performed using multivariable logistic and Cox regression and Kaplan-Meier.
Results
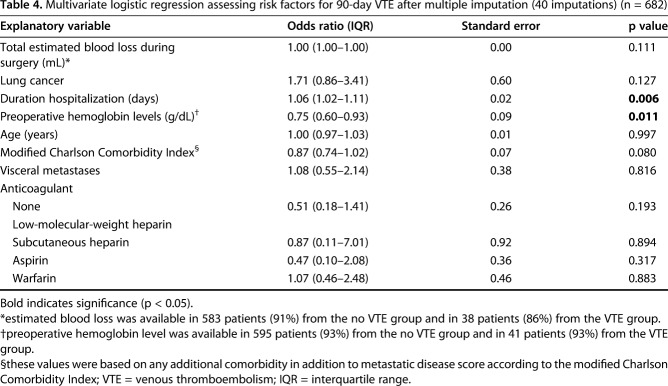
Overall, 6% (44 of 682) of patients had symptomatic VTE; 22 patients sustained a DVT, and 22 developed a PE. After controlling for relevant confounding variables, higher preoperative hemoglobin level was independently associated (odds ratio [OR], 0.75; 95% confidence interval [CI], 0.60–0.93; p = 0.011) with decreased symptomatic VTE risk, the presence of symptomatic VTE was associated with a worse 1-year survival rate (VTE: 27% [95% CI, 14%–40%] and non-VTE: 39% [95% CI, 35%–43%]; p = 0.041), and no association was found between wound complications and the use of chemoprophylaxis (OR, 3.29; 95% CI, 0.43–25.17; p = 0.252).
Conclusions
The risk of symptomatic 90-day VTE is high in patients undergoing surgery for long bone metastases. Further study would be needed to determine the VTE prevention strategy that best balances risks and benefits to address this complication.
Level of Evidence
Level III, therapeutic study.
Introduction
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolus (PE), is a major public health problem that affects 300,000 to 600,000 individuals in the United States each year and is accompanied by considerable mortality and morbidity [15, 43, 44, 49]. The combination of neoplastic disease and major orthopaedic surgery, both of which are known factors associated with VTE [1, 2, 9, 14, 18, 24, 27, 33, 35], might put patients at additional risk for developing VTE and could affect survival.
A previous study reported a symptomatic 90-day VTE rate of 10% in 10 of 306 patients undergoing surgery for nonspinal skeletal metastases [39]. However, important variables in the analysis were absent, including prior local radiotherapy/systemic chemotherapy known for their association with VTE. In addition, the current study examines the relationship between wound complication rate and chemoprophylaxis because this is particularly interesting in the context of the discussion regarding VTE prevention strategies [11, 26]. Other studies have also been limited by the size of the patient cohort(s) and the fact that they involved heterogeneous patient populations including both primary tumors and metastatic bone lesions [3, 10, 23, 25, 28, 30, 32, 34, 47]. Determining factors associated with postoperative VTE development and assessing survival consequences may identify high-risk patients who might benefit from intensified VTE prevention strategies. However, current chemoprophylaxis protocols from national guidelines present ambiguous recommendations about type, dosage, and duration after major orthopaedic surgery, let alone after surgery for long bone metastases [11, 26]. Accurately balanced chemoprophylaxis protocols are desired to balance between effectively preventing VTE and avoiding wound complications. Determining the relationship among chemoprophylaxis, the rate of symptomatic VTE, and wound complications would help clinical decision-making.
We therefore asked: (1) What proportion of patients develop symptomatic VTE after surgery for long bone metastases? (2) What factors are associated with the development of symptomatic VTE among patients receiving surgery for long bone metastases? (3) Is there an association between the development of symptomatic VTE and 1-year survival among patients undergoing surgery for long bone metastases? (4) Does chemoprophylaxis increase the risk of wound complications among patients undergoing surgery for long bone metastases?
Patients and Methods
Our institutional review board approved a waiver of informed consent for this retrospective study at the Massachusetts General Hospital and Brigham and Women's Hospital. The study included 682 patients 18 years of age or older who had a surgical procedure for impending or pathologic metastatic long bone fracture between 2002 and 2013. We considered the humerus, radius, ulna, femur, tibia, and fibula as long bones; metastatic disease was defined as metastases from solid organs, multiple myeloma, or lymphoma [29]. The patients included 383 (56%) women and 299 men (44%) with a median age of 64 years (interquartile range [IQR], 54–72; Table 1).
Table 1.
Patient and treatment characteristics for the no VTE and VTE groups (n = 682)
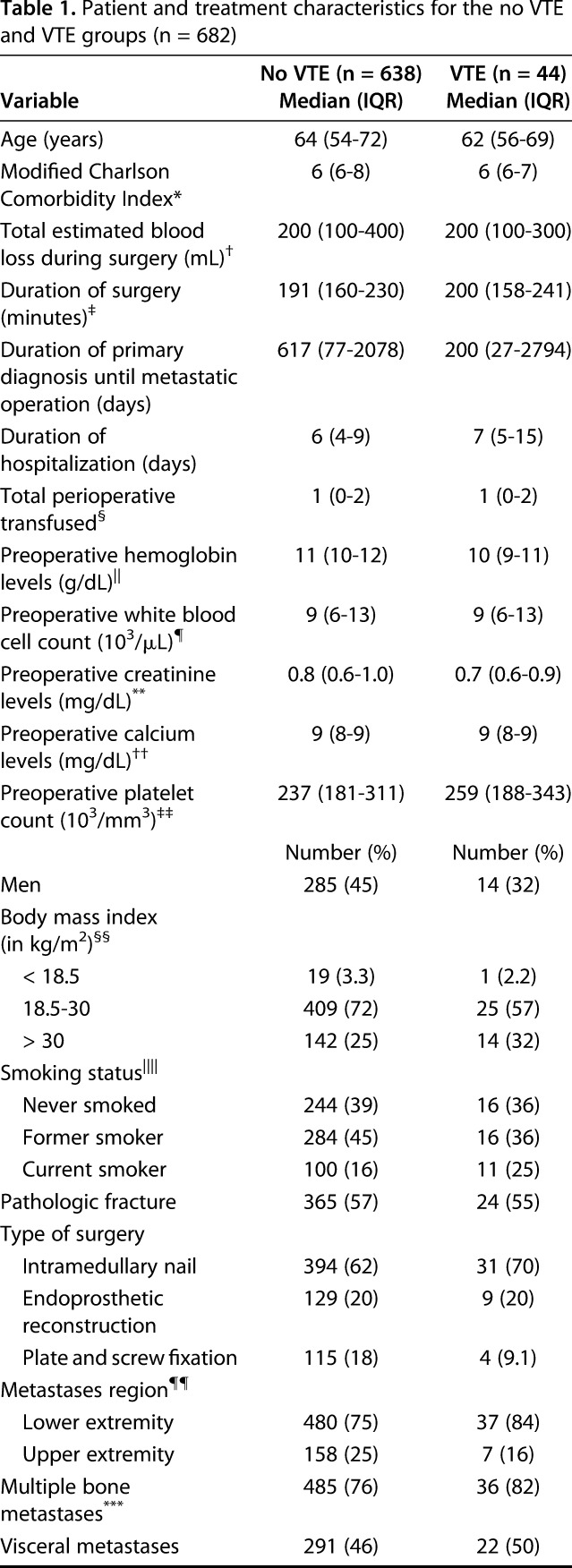

The median duration of surgery was 191 minutes (IQR, 160–230 minutes) and median hospitalization was 6 days (IQR, 4–9 days). There were 389 (57%) pathologic and 293 (43%) impending fractures. Of the 682 fractures, 492 (72%) involved the femur; 160 (23%) the humerus; 25 the tibia; and five the radius or ulna. Inferior vena cava (IVC) filters were placed in 17 patients: one (2.3%) in the VTE group and 16 (2.5%) in the non-VTE group. Most common primary tumor types included lung (24%), breast cancer (23%), and multiple myeloma (16%) (Table 2).
Table 2.
Origin of the primary tumor (n = 682)
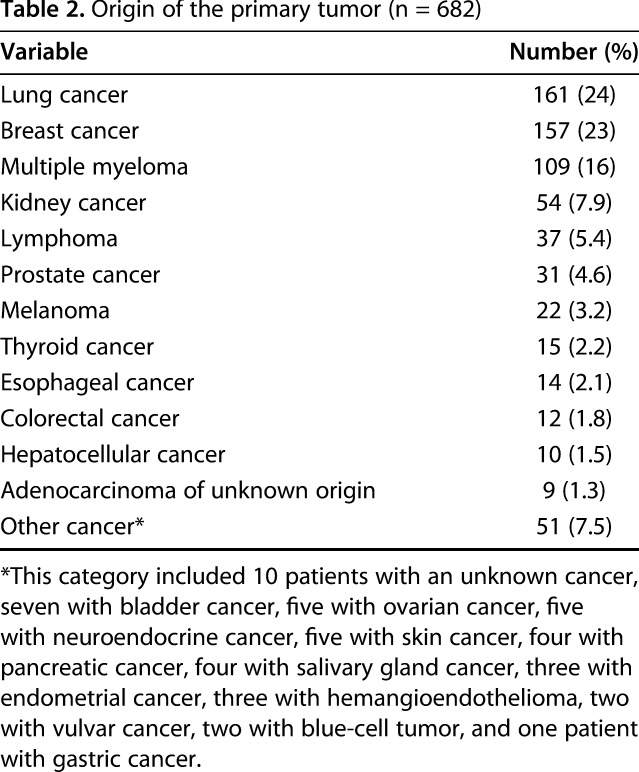
We excluded patients with (1) revision procedures, defined as any subsequent procedure after the index surgery addressing the metastatic lesion; (2) surgery resulting from metastatic fractures in multiple bones; (3) surgical treatment other than intramedullary nailing, plate-screw fixation, endoprosthetic reconstruction, or a combination; and (4) a diagnosed symptomatic VTE within 2 weeks before surgery because this would interfere with the main aim of the study to find factors associated with developing postoperative VTE. Medical records were flagged with diagnostic and billing codes for prophylactic treatment of an impending fracture or a pathologic long bone fracture and then manually checked for eligibility [16]. The surgeon selected the operating procedure based on primary tumor type, size and location of the metastatic lesion, estimated survival, and level of disability and pain. Postoperative care and rehabilitation varied based on differences in disease severity.
We obtained data through chart review by two independent research fellows (OQG, PTO). Our primary outcome was a symptomatic VTE, presenting with swelling, redness, or pain in the lower extremities or problems with breathing, defined as any symptomatic PE or symptomatic distal or proximal DVT within 90 days of surgery diagnosed with the following diagnostic procedures: venography, impedance plethysmography, pulmonary arteriography, chest CT, ventilation-perfusion lung scan, and vascular ultrasound. Our secondary outcome was survival after surgery. October 1, 2016, was considered the final date of followup for survival outcome assessment. We determined date of death by using the Social Security Index and medical charts. At followup after 90 days and 1 year, respectively, 4% (25 of 682) and 8% (53 of 682) were lost to followup. Our third outcome was documented wound complications, defined as a wound complication that might be attributable to chemoprophylaxis within 90 days of surgery; none of these was minor. These complications were nine deep infections treated with irrigation and débridement, five superficial wound complications consisting of three wound dehiscences that were treated surgically, and two hematomas that were treated without surgery, and four deep wound complications consisting of three hematomas and one retroperitoneal bleed treated surgically [38]. Wound complications such as wound inflammation requiring antibiotics were disregarded. Only two patients had a wound complication followed by a symptomatic VTE.
During the period in question, we generally used either 40 mg enoxaparin or 325 mg aspirin daily for patients operated on the lower extremity. For surgery on the upper extremity we used 325 mg aspirin daily for major reconstruction and no chemoprophylaxis for less invasive surgery. Other general thromboembolic prophylactic dosages used were: 5000 IUs dalteparin daily, warfarin dependent to maintain an international normalized ratio of 2.0:2.5, and 5000 IUs subcutaneous heparin every 12 hours. Patients on preoperative chemoprophylaxis continued their initial medication postoperatively. All chemoprophylaxis was started 6 to 12 hours after surgery and continued day to day but was discontinued if a bleeding complication developed. In case of contraindications for chemoprophylaxis, an IVC filter was placed before surgery on the lower extremity and no chemoprophylaxis was prescribed for surgery on the upper extremity. A total of 17 IVC filters were placed. Chemical anticoagulants, within a maximum range of 14 days postoperatively, were considered prophylactic. The most aggressive chemoprophylaxis regimen was considered in our analyses in case of overlapping regimens. The following anticoagulant regimens were used: low-molecular-weight heparin (LMWH) for 358 of 682 patients (52%); no form of chemical anticoagulant for 113 patients (17%); warfarin for 129 patients (19%); aspirin for 66 (10%) patients; and subcutaneous heparin for 16 patients (2%). Compression stockings and sequential compression devices were not included as potential variables because they were routinely used as mechanical prophylaxis at both centers in all patients after surgery throughout their hospitalization.
Preoperative laboratory values, nearest to surgery with a maximum range of 7 days, included hemoglobin level (g/dL), creatinine level (mg/dL), calcium level (mg/dL), white blood cell count (1000/mm3), and platelet count (1000/mm3). Fracture type was defined as pathologic or impending fracture. Impending fractures were considered imminent to pathologic fracture if they possessed a destructive bone lesion with no visible fracture line, loss of height, rotation, or angulation. The surgeon determined operative treatment, based on the severity of pain and the degree of destruction, to prevent a pathologic fracture. The patient comorbidity status was determined using the modified Charlson Comorbidity Index [8, 36]. An International Classification of Diseases, 9th Revision code-based algorithm classifying 12 comorbidities preoperatively provided a score ranging from 0 to 24 [36] with a higher score corresponding with a more severe comorbidity status [16]. Placement of an IVC filter was considered prophylactic when it was placed preoperatively or within 90 days postoperatively. IVC filters placed after VTE were disregarded. We determined operative treatment time in minutes using the anesthesia time as a surrogate marker, which measured the presence of the patient in the operating room from arrival until departure.
We used multivariable logistic regression analysis controlling for confounding variables identified in bivariate testing with a p value < 0.10 and presumed to be relevant to VTE [39, 41] to assess independent risk factors for symptomatic VTE. Odds ratios for continuous variables are interpreted in terms of each unit increase or decrease on the scale (ie, 1 to 2, 2 to 3, etc; that is, each one-unit increment of hemoglobin with an odds ratio of < 1 corresponds to a decreased risk of the outcome in question, in this case, symptomatic VTE). Bivariate analysis found that higher blood loss during surgery (odds ratio [OR], 1.00; 95% confidence interval [CI], 1.00–1.00; p = 0.036) and higher preoperative hemoglobin levels (OR, 0.74; 95% CI, 0.60–0.92; p = 0.007) were associated with decreased and longer duration of hospitalization (OR, 1.06; 95% CI, 1.02–1.10; p = 0.003) with increased risk of symptomatic VTE development (see Appendix, Supplemental Digital Content 1). Lung cancer (OR, 1.74; 95% CI, 0.91–3.34; p = 0.094) was included in the multivariable analysis as a result of a p value of < 0.1. Additional variables controlled for were age, the modified Charlson Comorbidity Index, visceral metastases, and chemoprophylaxis. Multivariate logistic regression was also used to assess the relation between chemoprophylaxis and wound complications controlling for age and the modified Charlson Comorbidity Index. We used Cox regression analysis after controlling for the confounding factors age, gender, body mass index (BMI), the modified Charlson Comorbidity Index, visceral and other bone metastases, estimated blood loss, operation type, and pathologic fracture to determine differences in survival between the symptomatic VTE and non-VTE groups. Kaplan-Meier plots demonstrated the survival curves for both groups. We applied multiple imputations to estimate missing values for estimated blood loss during surgery (61 of 682 patients) and preoperative hemoglobin levels (46 of 682 patients). A two-tailed p value < 0.05 was considered significant. All statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, TX, USA).
Results
Symptomatic VTE was diagnosed in 6% (44 of 682) of patients; 22 had a PE and 22 had a DVT (Table 3). The median age of the 44 patients was 62 years (IQR, 56–69 years), and 14 (32%) were men. Of the 22 patients with a PE, two had a confirmed DVT 1 day later, seven tested negative on CT or ultrasound, and 13 did not undergo assessment of DVT presence. One patient died 5 days postoperatively as a result of PE. Symptomatic VTE was diagnosed in six patients with metastatic multiple myeloma and two patients with lymphoma. More than half of the patients (57%) developed a symptomatic VTE after their postoperative discharge; the median postoperative hospitalization of these patients was 7 days (IQR, 5–15 days), and the median time between surgery and symptomatic VTE development was 12 days (IQR, 4–45 days, last symptomatic VTE event documented at 85 days after surgery).
Table 3.
90-Day VTE, wound complications, and anticoagulant use (n = 682)
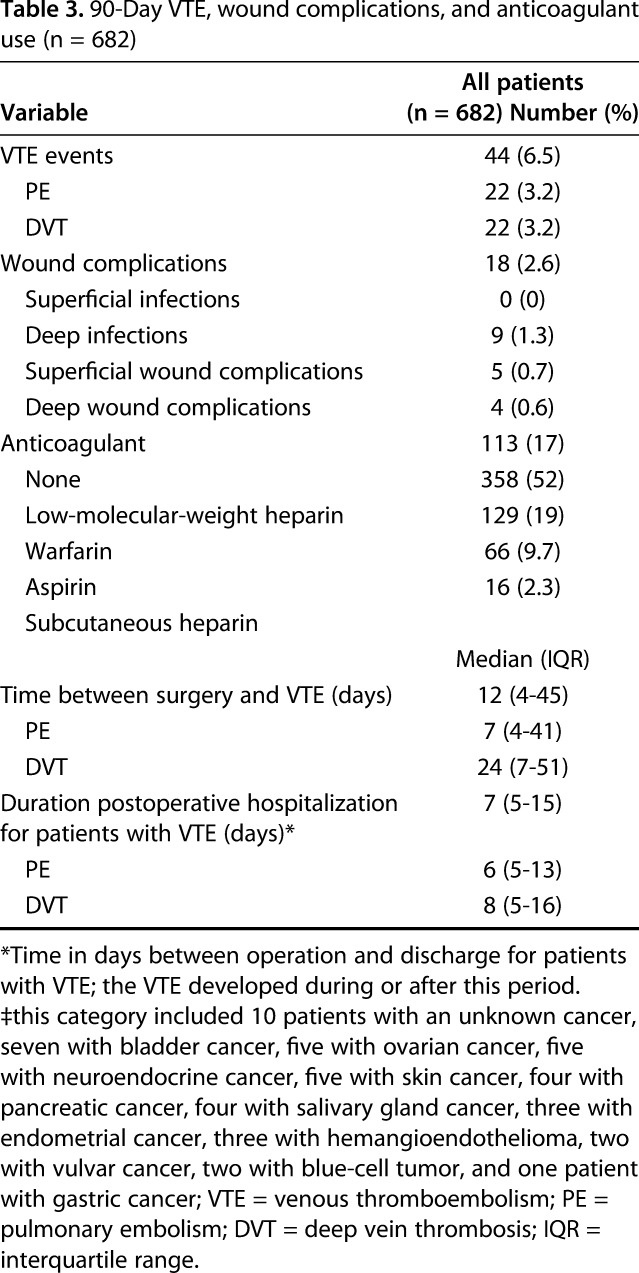
After controlling for potentially relevant confounding variables (Table 4), we found that the following two factors were independently associated with, respectively, increased and decreased risk of symptomatic VTE development: longer duration of hospitalization (OR, 1.06; 95% CI, 1.02–1.11; p = 0.006) and higher preoperative hemoglobin levels (OR, 0.75; 95% CI, 0.60–0.93; p = 0.011; Table 4). Symptomatic VTE occurred in 39 of 569 patients when considering the group that used any chemoprophylaxis and in five of 113 patients for no chemoprophylaxis, demonstrating no association after controlling for age, gender, the modified Charlson Comorbidity Index, and lung cancer histology (OR, 1.65; 95% CI, 0.63–4.32; p = 0.310). Patients who had a symptomatic VTE within 90 days had lower 1-year survival than did those without symptomatic VTE after controlling for the confounding variables: age, gender, BMI, fracture, the modified Charlson Comorbidity Index, visceral and other bone metastases, estimated blood loss, operation type, and pathologic fracture (27% [95% CI, 14%–40%] and 39% [95% CI, 35%–43%]; p = 0.041) (Fig. 1). The probability of developing a symptomatic VTE rose gradually with a notable increase at 30 days after surgery (Fig. 2). Timing of symptomatic VTE ranged from 1 day to 85 days.
Table 4.
Multivariate logistic regression assessing risk factors for 90-day VTE after multiple imputation (40 imputations) (n = 682)
Fig. 1.
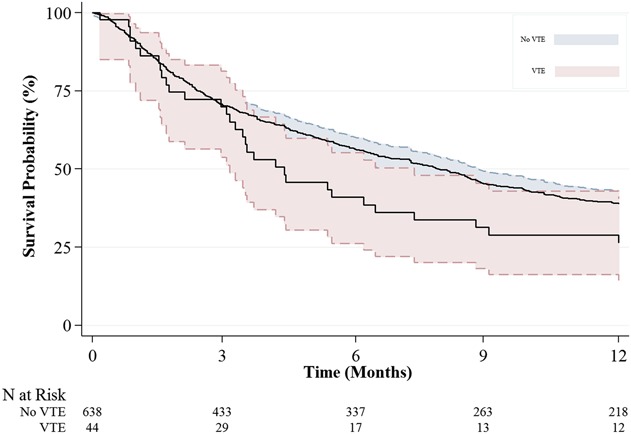
Kaplan-Meier plot demonstrating the survival probability with 95% CIs for patients with and without postoperative symptomatic VTE (p = 0.041).
Fig. 2.
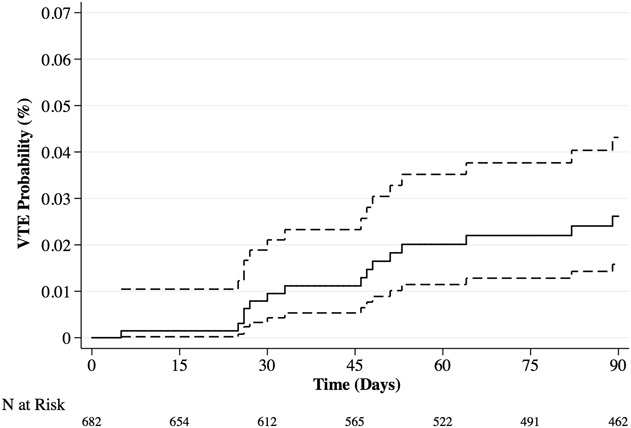
Kaplan-Meier plot demonstrating the probability of developing a symptomatic VTE within 90 days postoperatively 0.03% (95% CI, 0.02–0.04). The risk of symptomatic VTE occurrence has a sudden increase at 30 days postoperatively and keeps increasing gradually thereafter.
With the numbers available, we found no association, after controlling for age and the modified Charlson Comorbidity Index, between any of the studied chemoprophylaxis regimens and the occurrence of 18 wound complications, consisting of 10 (56%) for LMWH (reference value), six (33%) for warfarin (OR, 1.40; 95% CI, 0.47–4.19; p = 0.547), one (6%) for aspirin (OR, 0.54; 95% CI, 0.07–4.32; p = 0.563), one (6%) for no form of chemoprophylaxis (OR, 0.31; 95% CI, 0.04–2.43; p = 0.263), and zero for heparin (no values available). An additional subanalysis between chemoprophylaxis in its entirety and no chemoprophylaxis also showed no difference, but this was underpowered.
Discussion
Patients undergoing major surgery for bone metastases are at high risk for developing postoperative VTE. This study is advantaged over prior work considering the more than double sample of patients with long bone metastases and extensive followup in light of the cohort’s clinical characteristics adding more than 15 potential variables for analysis. The symptomatic VTE incidence was 6% and after controlling for potential confounding variables such as age and lung cancer histology, we found that preoperative hemoglobin levels and duration of hospitalization were independently associated with symptomatic VTE development. After controlling for confounding variables, patients with symptomatic VTE had worse survival, although only one of the 682 (0.1%) patients died from PE. There was no association between any of the studied anticoagulant regimens and the development of symptomatic VTE or postoperative wound complications. However, we were not sufficiently powered to address this problem.
This study has limitations. First, because of the retrospective study design, there was no uniform anticoagulant regimen or standardization regarding chemoprophylaxis use. In general, we used 40 mg enoxaparin daily for lower extremity surgery and 325 mg aspirin daily for lower or upper surgery. Second, uncontrolled for differences likely exist in the survival analysis between the symptomatic VTE and non-VTE groups, because only one fatal PE was identified. However, we controlled for multiple confounding survival variables such as age and the modified Charlson Comorbidity Index. Third, we could not confirm the exact duration and compliance of anticoagulant use for all patients because chemoprophylaxis duration during and after hospitalization was not recorded and postdischarge compliance was not monitored. However, both institutions maintained a protocol that required patients to use anticoagulants for 4 weeks postoperatively. Additionally, it was protocol that patients use sequential compression devices and compression stockings during postoperative hospitalization. Fourth, we included only symptomatic VTE because of the lack of a screening protocol, which likely resulted in an underestimated VTE incidence. We anticipate a relatively low number of clinically relevant VTEs were missed because this complicated patient population was closely monitored by healthcare providers and frequently visited the clinic postoperatively. Fifth, a history of VTE was not included in our analysis as a result of the unreliability of this specific personal history data in a tertiary center. Lastly, metastases from lymphoma and multiple myeloma were included, which are known for their increased symptomatic VTE risk and better prognosis [20]. Nonetheless, we included them because these metastases represent 21% (146 of 682) of the long bone metastases.
In this series, 6% of patients (44 of 682) developed symptomatic VTE and 3% (22 of 682) developed PE. This symptomatic 90-day VTE rate is within the reported symptomatic VTE range of 2.7% to 28% of comparable musculoskeletal metastases series [3, 10, 23, 25, 28, 30, 32, 39, 41, 47]. The Ratasvouri [39] study reported comparable results of a symptomatic 90-day VTE rate of 10% (30 of 306), PE rate of 3.3% (10 of 306), poor survival of patients with VTE, and the late onset of postoperative VTE development. In addition, higher preoperative hemoglobin was identified as a factor associated with decreased postoperative symptomatic VTE development and more than 15 potential variables were added to the analysis. Also, although underpowered, this study elaborated on the discussion regarding wound complication and chemoprophylaxis in the context of VTE prevention strategies.
Although the proportion of symptomatic VTE was considerable, for fatal PE, it is low, occurring in only one of 682 patients, meaning that these patients are not dying as a direct cause of symptomatic VTE. Thrombocytosis is an independent predictor of survival in multiple cancers [42, 45] through enhanced invasiveness of tumor cells [19], promotion of tumor cell motility [40], and stimulation of epithelial-mesenchymal transition [22]. However, the mechanism by which symptomatic VTE and nonfatal PE are associated with worse survival is poorly understood. A recent study demonstrated that activated platelets, which are seen during VTE, may inhibit the immune response to cancer cells by facilitating T lymphocyte inhibition through binding to transforming growth factor-β in serum. The study further postulated that antiplatelet therapy may be an effective adjunct to immunotherapy [37]. These data should be considered when considering chemoprophylaxis in the setting of surgery for metastatic disease. Another possible reason for the poor survival in patients with VTE is the highly complex patient population with multiple comorbidities and other disease-related factors, in which patients with more advanced cancer develop VTE more easily. Moreover, only one fatal PE was confirmed indicating that VTE may function more as a predictor than as the main cause for poor survival.
The lower extremity, and especially the femur, was the most common surgery site. Although lower extremity procedures have a greater effect on patient mobility, the location of surgery was not a factor associated with symptomatic VTE development in this study. It is possible that the risk caused by skeletal metastatic disease—malignancy promotes a hypercoagulable state—substantially outweighs the risk of immobility as a result of lower extremity surgery. A higher preoperative hemoglobin level was an independent factor associated with decreased symptomatic VTE. This biomarker was previously identified as a predictive marker for cancer-associated VTE [21]. Clinically, preoperative hemoglobin should be incorporated into the risk adjustment as a factor associated with postoperative symptomatic VTE.
Duration of hospitalization was also independently but only slightly associated with increased symptomatic VTE. We note that this association between longer duration of hospitalization and development of symptomatic VTE does not imply that longer hospitalization causes symptomatic VTE. It may well be the other way around: the occurrence of symptomatic VTE results in longer hospitalization. This is demonstrated by comparing different means of hospitalization. The means of hospitalization for all patients without VTE (7 ± 6 days) and patients with symptomatic VTE that developed during hospitalization (14 ± 6 days) are quite different, but this longer duration of hospitalization is preceded by early development of symptomatic VTE in this group (4 ± 3 days). Additionally, the means of hospitalization for all patients without VTE patients (7 ± 6 days) and those with symptomatic VTE that developed after discharge (8 ± 7 days) are nearly identical, resulting in no difference (see Appendix, Supplemental Digital Content 2). This indicates that the occurrence of symptomatic VTE during hospitalization may result in a longer hospitalization and that longer duration of hospitalization should not be considered a factor associated with symptomatic VTE development.
No association was found between a specific anticoagulant regimen and the development of wound complications. However, this analysis was underpowered to detect a relationship given the relatively low rate of wound complications within each separate anticoagulant group. Shallop et al. [41] reported a comparably low risk of wound complications and infection in intramedullary nailing for metastatic bone lesions as did a systematic review including 3211 metastatic lesions in the femur [17]. The risk of major wound complications seems low with only nine of 682 patients undergoing revision surgery for deep infection, three for a deep, large hematoma, and one patient who developed a large retroperitoneal hematoma and was admitted to the intensive care unit. Meanwhile, symptomatic VTE development occurs frequently in this population. Considering these results, future studies need to determine the relation between wound complications and various anticoagulant agents as well as the ideal prophylactic dosage to address the high rate of symptomatic VTE [41].
The probability of developing a symptomatic VTE increased precipitously 30 days postoperatively and steadily increased over a 90-day period (Fig. 2). Previous studies have shown that the risk of postoperative symptomatic VTE persists for several weeks after hospital discharge in patients undergoing high-risk orthopaedic surgery [4, 5, 7, 48]. Correspondingly, most symptomatic VTEs, which occurred in 25 of 44 patients (57%), developed after discharge; for patients with symptomatic VTE, the time in days between surgery and symptomatic VTE (median, 12 days; IQR, 4–45 days) was considerably longer than the duration of postoperative hospitalization (median, 7 days; IQR, 5–15 days). The onset of late postoperative symptomatic VTE was also observed in comparable studies, and it has been suggested that longer duration of prophylaxis may prevent this [41, 46]. However, most major national orthopaedic guidelines remain unclear about the duration of anticoagulant use, stating that patients and physicians should discuss the duration of prophylaxis [26]. Although this study was not designed to specifically address this compliance variable, previous studies report poor compliance of outpatient anticoagulant use and inappropriate prophylaxis prescription at discharge [6, 13, 50]. Moreover, given the tendency toward shorter hospitalization after major orthopaedic surgery, the importance of compliance of outpatient anticoagulants in preventing postoperative symptomatic VTE must be stressed [31]. Novel oral anticoagulants could fulfill a prominent role, because most patients prefer oral agents [12]. Further study should elucidate the ideal duration of a postoperative prophylactic regimen. Interestingly, 10 of the 22 patients with symptomatic DVT (45%) had a DVT away from the site of surgery; five patients (23%) had DVTs isolated to the contralateral limb and five patients (23%) had bilateral DVTs. This supports the concept that systemic factors stimulate thrombosis in patients with cancer in addition to local and mechanical factors.
In conclusion, our study presents a high symptomatic 90-day VTE rate among patients undergoing surgery for long bone metastases, warranting several considerations. First, protocols may need to incorporate patient-specific risk factors such as preoperative hemoglobin levels. Second, future studies should elucidate the ideal postoperative VTE prevention regimen. In concordance with similar studies, the risk for VTE is clearly high, requiring further investigation.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution waived approval for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Amato A, Pescatori M. Perioperative blood transfusions and recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;1:CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behranwala KA, Williamson RC. Cancer-associated venous thrombosis in the surgical setting. Ann Surg. 2009;249:366–375. [DOI] [PubMed] [Google Scholar]
- 3.Benevenia J, Bibbo C, Patel DV, Grossman MG, Bahramipour PF, Pappas PJ. Inferior vena cava filters prevent pulmonary emboli in patients with metastatic pathologic fractures of the lower extremity. Clin Orthop Relat Res. 2004;426:87–91. [DOI] [PubMed] [Google Scholar]
- 4.Bergqvist D. The postdischarge risk of venous thromboembolism after hip replacement. The role of prolonged prophylaxis. Drugs. 1996;52(Suppl 7):55–59. [DOI] [PubMed] [Google Scholar]
- 5.Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 6.Bergqvist D, Arcelus JI, Felicissimo P. Evaluation of the duration of thromboembolic prophylaxis after high-risk orthopaedic surgery: the ETHOS observational study. Thromb Haemost. 2012;107:270–279. [DOI] [PubMed] [Google Scholar]
- 7.Bergqvist D, Lindblad B. A. 30-year survey of pulmonary embolism verified at autopsy: an analysis of 1274 surgical patients. Br J Surg. 1985;72:105–108. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 9.Clagett GP, Anderson FA, Jr, Geerts W, Heit JA, Knudson M, Lieberman JR, Merli GJ, Wheeler HB. Prevention of venous thromboembolism. Chest. 1998;114:531S–560S. [DOI] [PubMed] [Google Scholar]
- 10.Damron TA, Wardak Z, Glodny B, Grant W. Risk of venous thromboembolism in bone and soft-tissue sarcoma patients undergoing surgical intervention: a report from prior to the initiation of SCIP measures. J Surg Oncol. 2011;103:643–647. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S–e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman RJ. Novel oral anticoagulants for VTE prevention in orthopedic surgery: overview of phase 3 trials. Orthopedics. 2011;34:795–804. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Long A, Xie Z, Meng Y, Tan J, Lv H, Zhang L, Zhang L, Tang P. The compliance of thromboprophylaxis affects the risk of venous thromboembolism in patients undergoing hip fracture surgery. Springerplus. 2016;5:1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:381S–453S. [DOI] [PubMed] [Google Scholar]
- 15.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815. [DOI] [PubMed] [Google Scholar]
- 16.Janssen SJ, Braun Y, Ready JE, Raskin KA, Ferrone ML, Hornicek FJ, Schwab JH. Are allogeneic blood transfusions associated with decreased survival after surgery for long-bone metastatic fractures? Clin Orthop Relat Res. 2015;473:2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen SJ, Teunis T, Hornicek FJ, van Dijk CN, Bramer JAM, Schwab JH. Outcome after fixation of metastatic proximal femoral fractures: a systematic review of 40 studies. J Surg Oncol. 2016;114:507–519. [DOI] [PubMed] [Google Scholar]
- 18.Karadimas EJ, Papadimitriou G, Theodoratos G, Papanikolaou A, Maris J. The effectiveness of the antegrade reamed technique: the experience and complications from 415 traumatic femoral shaft fractures. Strateg Trauma Limb Reconstr. 2009;4:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, Nishimura T, Asakura H, Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana A, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin PP, Graham D, Hann LE, Boland PJ, Healey JH. Deep venous thrombosis after orthopedic surgery in adult cancer patients. J Surg Oncol. 1998;68:41–47. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin DF, Wade CE, Champion HR, Salinas J, Holcomb JB. Thromboembolic complications following trauma. Transfusion. 2009;49:256S–263S. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SY, Lingard EA, Kesteven P, McCaskie AW, Gerrand CH. Venous thromboembolism in patients with primary bone or soft-tissue sarcomas. J Bone Joint Surg Am. 2007;89:2433–2439. [DOI] [PubMed] [Google Scholar]
- 26.Mont MA, Jacobs JJ, Boggio LN, Bozic KJ, Della Valle CJ, Goodman SB, Lewis CG, Yates AJ, Jr, Walters WC, 3rd, Turkelson CM, Wies JL, Donnelly P, Patel N, Sluka P; AAOS. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19:768–776. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery KD, Geerts WH, Potter HG, Helfet DL. Thromboembolic complications in patients with pelvic trauma. Clin Orthop Relat Res. 1996;329:68–87. [DOI] [PubMed] [Google Scholar]
- 28.Morii T, Mochizuki K, Tajima T, Aoyagi T, Satomi K. Venous thromboembolism in the management of patients with musculoskeletal tumor. J Orthop Sci. 2010;15:810–815. [DOI] [PubMed] [Google Scholar]
- 29.Nathan SS, Healey JH, Mellano D, Hoang B, Lewis I, Morris CD, Athanasian EA, Boland PJ. Survival in patients operated on for pathologic fracture: Implications for end-of-life orthopedic care. J Clin Oncol. 2005;23:6072–6082. [DOI] [PubMed] [Google Scholar]
- 30.Nathan SS, Simmons KA, Lin PP, Hann LE, Morris CD, Athanasian EA, Boland PJ, Healey JH. Proximal deep vein thrombosis after hip replacement for oncologic indications. J Bone Joint Surg Am. 2006;88:1066–1070. [DOI] [PubMed] [Google Scholar]
- 31.OECD. OECD Health Data 2009—comparing health statistics across OECD countries—OECD. Available at: http://www.oecd.org/health/oecdhealthdata2009comparinghealthstatisticsacrossoecdcountries.htm. Accessed January 15, 2018.
- 32.Ogura K, Yasunaga H, Horiguchi H, Ohe K, Kawano H. Incidence and risk factors for pulmonary embolism after primary musculoskeletal tumor surgery. Clin Orthop Relat Res. 2013;471:3310–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owings JTJ, Gosselin R. Acquired antithrombin deficiency following severe traumatic injury: rationale for study of antithrombin supplementation. Semin Thromb Hemost. 1997;23:17–24. [PubMed] [Google Scholar]
- 34.Patel AR, Crist MK, Nemitz J, Mayerson JL. Aspirin and compression devices versus low-molecular-weight heparin and PCD for VTE prophylaxis in orthopedic oncology patients. J Surg Oncol. 2010;102:276–281. [DOI] [PubMed] [Google Scholar]
- 35.Planès A, Vochelle N, Fagola M. Total hip replacement and deep vein thrombosis. A venographic and necropsy study. J Bone Joint Surg Br. 1990;72:9–13. [DOI] [PubMed] [Google Scholar]
- 36.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. [DOI] [PubMed] [Google Scholar]
- 37.Rachidi S, Metelli A, Riesenberg B, Wu BX, Nelson MH, Wallace C, Paulos CM, Rubinstein MP, Garrett-Mayer E, Hennig M, Bearden DW, Yang Y, Liu B, Li Z. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci Immunol. 2017;2:eaai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramo BA, Griffin AM, Gill CS, McDonald DJ, Wunder JS, Ferguson P, Bell RS, Phillips SE, Schwartz HS, Holt GE. Incidence of symptomatic venous thromboembolism in oncologic patients undergoing lower-extremity endoprosthetic arthroplasty. J Bone Joint Surg Am. 2011;93:847–854. [DOI] [PubMed] [Google Scholar]
- 39.Ratasvuori M, Lassila R, Laitinen M. Venous thromboembolism after surgical treatment of non-spinal skeletal metastases—an underdiagnosed complication. Thromb Res. 2016;141:124–128. [DOI] [PubMed] [Google Scholar]
- 40.Schumacher D, Strilic B, Sivaraj K, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. [DOI] [PubMed] [Google Scholar]
- 41.Shallop B, Starks A, Greenbaum S, Geller DS, Lee A, Ready J, Merli G, Maltenfort M, Abraham JA. Thromboembolism after intramedullary nailing for metastatic bone lesions. J Bone Joint Surg Am. 2015;97:1503–1511. [DOI] [PubMed] [Google Scholar]
- 42.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. [DOI] [PubMed] [Google Scholar]
- 43.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 1998;158:585. [DOI] [PubMed] [Google Scholar]
- 44.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, Becker RC, Goldberg RJ. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. [DOI] [PubMed] [Google Scholar]
- 46.Sweetland S, Green J, Liu B, Berrington de Gonzalez A, Canonico M, Reeves G, Beral V. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583–b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuy B, Bhate C, Beebe K, Patterson F, Benevenia J. IVC filters may prevent fatal pulmonary embolism in musculoskeletal tumor surgery. Clin Orthop Relat Res. 2009;467:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007;89:799–807. [DOI] [PubMed] [Google Scholar]
- 49.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298–305. [DOI] [PubMed] [Google Scholar]
- 50.Wilke T, Müller S. Nonadherence in outpatient thromboprophylaxis after major orthopedic surgery: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2010;10:691–700. [DOI] [PubMed] [Google Scholar]