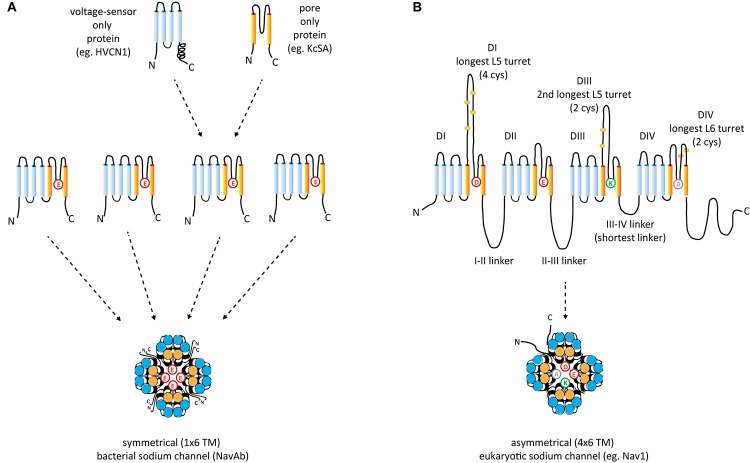
FIGURE 2.
Structural comparisons between (A) symmetrical, 1 × 6 TM (TM = transmembrane) homotetrameric, bacterial Na channels consisting of four repeat subunit (B) asymmetrical, eukaryotic four domain calcium and sodium channels consisting of 24 transmembrane segments (4 × 6 TM). Each of the four sodium and calcium channel domains consist of a voltage-sensor domain of four transmembrane segments, S1–S4 (blue), akin to voltage sensor only protein (e.g., HVCN1) and a pore domain, S5–S6 (orange), resembling the size of the two membrane segments of the inward rectifying K channels (e.g., KcsA). A dramatic difference between the 1 × 6 TM bacterial sodium channels and 4 × 6 TM calcium and sodium channels are the larger sizes of extracellular turrets rising before the pore selectivity filter, especially L5I and L5III which range from 40 to 105 amino acids long. The longest extracellular turret in Domain IV in 4 × 6 TM channels is always the extracellular turret rising after the pore selectivity filter (L6IV), while L5IV (the extracellular loop just before the pore selectivity filter) is always short. Comparatively speaking, 1 × 6 TM channels all possess short extracellular turrets of 10–15 amino acids long. Eight core, conserved cysteines populate the L5I - L5II - L5III - L6IV extracellular turrets in a 4-0-2-2 configuration in all eukaryotic 4 × 6 TM channels. Also novel are singleton N- and C-termini, and cytoplasmic linkers, of which the III-IV linker is almost always 53–57 amino acids long in eukaryotic 4 × 6 TM channels.