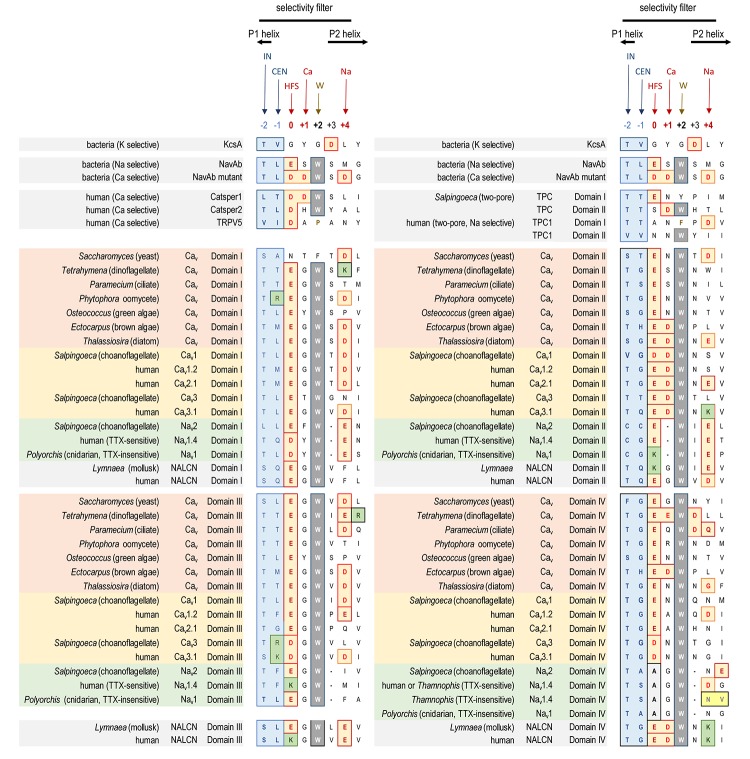
FIGURE 4.
Alignment of amino acid sequences contributing to the pore selectivity filters in representative calcium, sodium and NALCN channel homologs from single cell protists, yeast, algae, invertebrate and human, as well as 2 × 6 TM TPC channels, 1 × 6 TM channels (TRP, Catsper, and bacterial Na), and 1 × 2 TM channels (bacterial K). The pores of 4 × 6 TM channels from brown and green algae, yeast, single-cell choanoflagellates, cnidarian and mollusks are illustrated alongside human representatives, to illustrate the relationship of pore selectivity filters in different life forms. The selectivity filter is flanked by a descending P1 helix and ascending P2 helix. Residues contributing to the central (CEN) and inner (IN) sites of the selectivity filter are highlighted blue. Negative charged residues contributing to the selectivity filters (HFS sites), and to the ring of outer carboxylates are a red/brown color. A positively charged lysine residue (green color) populate the HFS site in Domain II or III of all Nav1 channels. The aspartate (D) residue in Domain II at the Ca site, next to the HFS site, is conserved in Cav1, Cav2 and Cav3 channels. Nav2 and Nav1 channels are shortened in the pore selectivity filter in Domain II and lack the Ca site altogether. Instead, a glutamate (E) residue is conserved at the Na site (HFS+4) in Domain II of Nav2 and Nav1 channels. Exceptionally conserved tryptophans (W site) form inter-repeat hydrogen bonds that stabilize the pore loop region. Outer ring carboxylates (Na site, especially in Domain IV) contribute to TTX sensitivity, which when altered lowers TTX insensitivity. Note the amino acid changes (yellow colored residues) in Domain IV of the TTX-sensitive hNav1.4 and TTX-low sensitive channels from garter snake (Thamnophis sirtalis) that adapts to feed on TTX-ladened newts by neutralizing a negative charge at the Na site. Bacterial Na channel, NavAb becomes calcium-selective with aspartate (D) substitutions at the HFS, Ca and Na sites. Amino acid sequences were aligned using MUSCLE 3.7 (Edgar, 2004) within EMBL-EBI web interface (Chojnacki et al., 2017).