Abstract
Background
Robotic-assisted THA has been promoted as potentially advantageous due to the precision it may afford when machining the proximal femur. However, few reports have compared the long-term clinical results of robotic techniques for femoral component insertion during THA regarding clinical outcomes scores or loosening.
Questions/purposes
The purpose of this study was to compare results from a randomized clinical trial (RCT) at a minimum followup of 10 years between robot-assisted and hand-rasped stem implantation techniques with regard to (1) Japanese Orthopaedic Association (JOA) clinical outcomes scores, and (2) aseptic loosening, revision surgery, and heterotopic ossification.
Methods
This is a concise followup of a previously reported RCT. In that trial, robot-assisted primary THA was performed on 75 hips (69 patients), and a hand-rasping technique was used on 71 hips (61 patients). Five experienced surgeons at two institutions participated in this trial; all THAs were performed through the posterolateral approach and the patients were treated similarly apart from the method used to prepare the femur. In all, 115 of 130 (88%) of patients initially randomized were available for followup at a minimum of 10 years (mean, 135 months; range, 120–152 months). There was no differential loss to followup between the study groups, and the final study groups here included 64 hips in 59 patients in the robotic group, and 64 hips in 56 patients in the hand-milling group. There were no differences between the study groups in terms of age, sex, diagnosis, body-mass index, or baseline JOA scores. The primary study endpoint was the JOA score, which is scored from 0 to 100, with higher scores representing better function and less pain. Secondary outcomes were revision surgery, and radiographic signs of aseptic loosening and heterotopic ossification as assessed using the four-grade Brooker scale by individuals other than the operating surgeon.
Results
At a minimum of 10 years postoperatively, there were no differences between patients treated with robot-assisted surgery or hand rasping in JOA scores (97 ± 5 versus 96 ± 7, mean difference 1.4; p = 0.159). No stems in either group developed aseptic loosening, and there were no revisions in either group. There was no difference between the groups in heterotopic ossification (19 of 64 [30%] in the robot-assisted group versus 12 of 64 [19%] in the hand-rasping group; p = 0.186), severe heterotopic ossification was uncommon in both groups, and no hips developed Grade 4 heterotopic ossification in either group.
Conclusions
Clinically and radiographically, THAs performed with robotic milling for stem implantation did not result in better 10-year clinical outcomes scores, or a lower risk of loosening or revision, compared with hand-rasping. We recommend against widespread adoption of robotic milling for stem implantation in primary cementless THAs.
Level of Evidence
Level II, therapeutic study.
Introduction
The robotic system, ROBODOC®, (THINK Surgical Inc, Fremont, CA, USA) was, to our knowledge, the first active robot designed to reduce human errors in the implantation of femoral components during cementless THA [2]. Clinical use of this system began in 1992 [18]. In 2008, the FDA granted the system 510(k) clearance for THA procedures. Several short-term clinical studies using this system have been published, most of which suggested that ROBODOC seemed to offer short-term advantages in terms of clinical scores, implant fit, intraoperative fracture risks, and pulmonary embolic events [2, 10, 12, 16]. We previously reported results after a 5-year followup period [15] using this robotic system, and at that time, we found less variance in limb-length inequality and less stress shielding of the proximal femur, although clinical outcomes scores were not different.
However, to our knowledge, there has been only one report of longer-term followup studies evaluating the performance of THAs implanted using this robotic-assisted system [3]; this report, which was the work of a designer of the robotic system in question, found small differences in some outcomes scores favoring the robot-assisted approach, and no differences in survivorship between robotic-assisted and conventional THA. A substantial number of patients in that report were lost to followup.
We therefore sought to review at 10 years the results from our earlier randomized trial [15], which compared robot-assisted and hand-rasped stem implantation techniques. Specifically, we sought to compare results from a randomized clinical trial (RCT) at a minimum followup of 10 years between robot-assisted and hand-rasped stem implantation techniques in terms of (1) Japanese Orthopaedic Association (JOA) clinical outcomes scores, and (2) aseptic loosening, revision surgery, and heterotopic ossification.
Methods
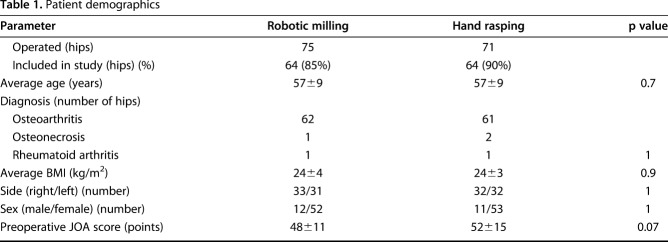
This is a concise followup of a previously reported RCT [15]. The study was approved by our institutional review board. Randomization was performed by a person (SN) not involved in the study; randomization was generated using a table of random numbers, and allocation was performed using sealed, opaque envelopes. The two groups (robotic-milling and hand-rasping) were concurrent; however, because the robotic-milling procedure needed prior pin implantation when this study was performed, the patients could not be blinded for the surgery. Currently, a nonpin-based surface registration technique has been developed and used in this system. Between 2000 and 2002, we performed 146 primary THAs on 130 patients. Robot-assisted primary THA was performed on 75 hips (69 patients), and a hand-rasping technique was used on 71 hips (61 patients). Among them, 11 hips (10 patients) in the robotic-milling group and seven hips (five patients) in the hand-rasping group were lost to followup. Thus, this study included 115 patients who had a total of 128 THAs (64 hips of 59 patients in the robotic-milling group, 64 hips of 56 patients in the hand-rasping group), and these individuals were followed for at least 10 years. A total of 59 of 69 patients (86%) in the robot-assisted group and 56 of 61 patients (92%) in the hand-rasping group were studied here. The mean followup was 135 months (range, 120-152 months). There were no differences between the two groups in the distribution of patient age, diagnosis, BMI, gender, or preoperative Japanese Orthopaedic Association (JOA) hip score [20] (Table 1).
Table 1.
Patient demographics
In this series, we used pin-based registration for the robot-assisted THA procedure [15]. Briefly, implantation of two locator pins in the femur was performed the day before surgery. A CT scan was then carried out. Using the data, we planned the position and size of the VerSys® FM Taper stem (Zimmer, Warsaw, IN, USA) in 3-D on the ORTHODOC® workstation (THINK Surgical Inc). We described detailed methods of preoperative planning in our previous report [17]. During surgery, after loading the patient data into the robot, the surgeon exposed the pins, secured the patient’s leg, and performed pin-based registration of the femur. The surgeon milled the femur and then manually inserted the implant. A 26-mm femoral head was used for all patients in both groups. About half of the femoral heads in each group were cobalt-chrome and the rest were zirconia. In the hand-rasping group, patients underwent a preoperative CT scan, and the surgeon performed the same preoperative planning procedure as in the robotic-milling group. This allowed us to compare these two approaches to femoral canal preparation.
During surgery, the surgeon performed conventional manual hand-rasping, using a posterolateral approach. Five senior surgeons (NN, NS, TN, AK, HM) were involved in both procedures. Postoperatively, full weightbearing was permitted as early as possible.
The primary study endpoint was the JOA score. The JOA score has a maximum of 100 points, of which a pain score (0-40 points), a ROM score (0-20), a walking ability score (0-20), and an ADL score (0-20) are included. The best score is 100 points, and to our knowledge, no minimum clinically important difference (MCID) has been defined for this scoring system. Secondary outcomes were revision surgery, and radiographic signs of aseptic loosening [7] and heterotopic ossification as assessed using the four-grade Brooker scale [4] by a surgeon other than the operating surgeon.
For statistical analyses, we used the unpaired t-test to compare age and BMI, the Mann-Whitney U test to compare JOA scores, the Fisher’s exact test to compare surgical side and gender, and the Mann-Whitney exact test to compare Engh’s grade and Brooker’s grade.
Because there is no known MCID for the JOA score, and we had no similar previous study for comparison, we chose an effect size of 5 out of 100 points (our impression of a clinically important difference) on the JOA score as the difference we wished to be able to detect at 80% power and p < 0.05. This sample-size calculation determined that 65 hips in each group would be sufficient to determine the difference.
Differences were considered significant when the p value was less than 0.05. Statistical analyses were performed using SPSS 9.0 for Windows (SPSS, Chicago, IL, USA).
Results
At a minimum of 10 years postoperatively, there were no differences between patients treated with robot-assisted surgery or hand rasping in terms of JOA scores (97 ± 5 versus 96 ± 7, mean difference 1.4; p = 0.159).
No stems in either group developed aseptic loosening, and there were no revisions in either group. There was no difference between the groups in terms of heterotopic ossification (30% in the robot-assisted group, 19 of 64, versus 19% in the hand-rasping group, 12 of 64; p = 0.186), severe heterotopic ossification was uncommon in both groups, and no hips developed Grade 4 heterotopic ossification in either group (Table 2). No further complications were reported since the 5-year followup period [15].
Table 2.
Distribution of heterotopic ossification according to Brooker’s grading
Discussion
One of the issues in robot-assisted THA has been that there have been very few long-term followup studies demonstrating its utility [14]. While early studies of robotic-assisted THA suggested there may be some benefits associated with its use [2, 15, 16], the only other long-term study of which we are aware [3] was performed by a designer of the technology. That study found only very small differences in favor of use of robotic-assisted THA. Given the great expense associated with surgical robotics, we felt it important to present the 10-year results of our randomized trial on the topic. We found no benefit to the use of robot-assisted THA in terms of clinical outcomes scores, loosening, revision, or heterotopic ossification.
A limitation of this study is that because the robotic-milling procedure needs prior pin implantation, the patients could not be blinded for the surgery. This might have influenced the JOA score in favor of the robotic-milling group. Another limitation is that 12% of the patients were lost to followup, but because there was no differential loss to followup, we believe this issue is unlikely to influence the robustness of the main findings, although it raises a question of statistical power. We calculated that 65 hips in each group should have provided 80% power to detect a difference of 5 points on the JOA score [15]; with loss to followup, we had 64 in each group. Insofar as there is no published MCID for the JOA score, a reader can simply consider that there is a small chance that a difference as large as 5 points could have been present but not detected by our report. Larger differences, of course, would likely have been detected if present.
We found no differences between patients treated with robot-assisted THA and hand rasping in terms of JOA scores. This is comparable to the recent study by Bargar et al. [3], who found very small differences favoring the robotic-assisted group regarding several outcomes scores; in fact, those differences were smaller than the minimum clinically important differences (MCIDs) for the WOMAC and Harris hip scores. Ultimately, it is not the p value that matters, but the effect size; our study found no differences in JOA scores, and the only other long-term report of which we are aware [3], as noted above, found differences that patients were unlikely to perceive as clinically important.
We found no differences in loosening, revision surgery, or heterotopic ossification between our study groups. The other long-term study likewise showed no radiographic benefits in favor of robotic-assisted THA compared with the traditional approach, such as differences in loosening, osteolysis, or stress shielding [3]. In our earlier study we found more stress shielding of the proximal femur according to the Engh and Bobyn classifications [6] in the hand-rasping group compared with the robotic-milling group at 2 years postoperatively, and the difference was greater at 5 years [15]. However, as Engh et al. [8] reported later, stress shielding assessment on plain radiographs is not reliable; to our knowledge, unless it is quite severe, it generally is not clinically important and generally does not result in revision surgery [5, 9, 19]. We therefore abandoned the radiographic assessment of stress shielding in this study. Instead, we are planning to perform a dual energy X-ray absorptiometry (DEXA) study to see if there are any important differences; in a previous study we reported that when using DEXA, less bone loss occurred at the proximal periprosthetic areas in the robotic-milling group than hand-rasping group at 2 years postoperatively [11]. Again, if such differences are present, they seem not to be clinically important insofar as there were no between-group differences in terms of hip scores, loosening, or revision risk.
Whether robotic surgery will be accepted into common orthopaedic practice depends on its cost effectiveness [13]. Considering the high initial cost for ROBODOC—reported to be more than USD 600,000 [1]—and the fact that patients need pin implantation and a CT scan before THA, robotic milling for stem implantation would need to result in improved clinical scores as well as lower revision and complication rates to justify those front-end expenses. Our results showed no advantage favoring robotics for stem implantation at 10 years. As such, we cannot justify the expenses associated with robotic THA surgery for stem implantation on the basis of our findings.
We speculate that one reason for the absence of a finding of difference here is the improvement of cementless stems over the decades. During the 1980s, the clinical results using cementless stems were inconsistent because of bone ingrowth failure and persistent thigh pain. Manual preparation of the femoral cavity was considered a major cause of the problem. This spurred the development of a surgical robot to mill the femoral canal [18]. However, subsequent improvements in stem design, materials, ingrowth surface and surgical techniques have made cementless femoral fixation highly successful [21, 22]. These improvements may have minimized the benefit of the robotic-milling procedure.
In conclusion, we found no clinical or radiographic benefit to robotic milling for stem implantation compared with hand rasping during THA at a minimum followup of 10 years. As a result, we recommend against widespread adoption of robotic milling for stem implantation in primary cementless THA.
Acknowledgments
The authors thank the following individuals who have contributed to this work: Dr. Shunsaku Nishihara for conducting the randomization and analyzing the patients’ data, Dr. Takashi Nishii, Dr. Akihiro Kakimoto and Dr. Hidenobu Miki for participating in surgery and providing patients’ data.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Center of Arthroplasty, Kyowakai Hospital, Osaka, Japan.
References
- 1.Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31–36. [PubMed] [Google Scholar]
- 2.Bargar WL, Bauer A, Borner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res. 1998;354:82–91. [DOI] [PubMed] [Google Scholar]
- 3.Bargar WL, Parise CA, Hankins A, Marlen NA, Campanelli V, Netravali NA. Fourteen year follow-up of randomized clinical trials of active robotic-assisted total hip arthroplasty. J Arthroplasty. 2018;33:810–814. [DOI] [PubMed] [Google Scholar]
- 4.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 5.Bugbee WD, Culpepper WJ, 2nd, Engh CA, Jr., Engh CA., Sr. Long-term clinical consequences of stress-shielding after total hip arthroplasty without cement. J Bone Joint Surg Am. 1997;79:1007–1012. [DOI] [PubMed] [Google Scholar]
- 6.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. [DOI] [PubMed] [Google Scholar]
- 7.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants. The femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 8.Engh CA, Jr., McAuley JP, Sychterz CJ, Sacco ME, Engh CA., Sr. The accuracy and reproducibility of radiographic assessment of stress-shielding. A postmortem analysis. J Bone Joint Surg Am. 2000;82:1414–1420. [DOI] [PubMed] [Google Scholar]
- 9.Engh CA, Jr., Young AM, Engh CA, Sr., Hopper RH., Jr. Clinical consequences of stress shielding after porous-coated total hip arthroplasty. Clin Orthop Relat Res. 2003;417:157–163. [DOI] [PubMed] [Google Scholar]
- 10.Hagio K, Sugano N, Takashina M, Nishii T, Yoshikawa H, Ochi T. Effectiveness of the ROBODOC system in preventing intraoperative pulmonary embolism. Acta Orthop Scand. 2003;74:264–269. [DOI] [PubMed] [Google Scholar]
- 11.Hananouchi T, Sugano N, Nishii T, Nakamura N, Miki H, Kakimoto A, Yamamura M, Yoshikawa H. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25:1062–1069. [DOI] [PubMed] [Google Scholar]
- 12.Honl M, Dierk O, Gauck C, Carrero V, Lampe F, Dries S, Quante M, Schwieger K, Hille E, Morlock MM. Comparison of robotic-assisted and manual implantation of a primary total hip replacement. A prospective study. J Bone Joint Surg Am. 2003;85:1470–1478. [DOI] [PubMed] [Google Scholar]
- 13.Jacofsky DJ, Allen M. Robotics in arthroplasty: A comprehensive review. J Arthroplasty. 2016;31:2353–2363. [DOI] [PubMed] [Google Scholar]
- 14.Karthik K, Colegate-Stone T, Dasgupta P, Tavakkolizadeh A, Sinha J. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Joint J. 2015;97:292–299. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468:1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21:957–966. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara S, Sugano N, Nishii T, Tanaka H, Yoshikawa H, Ochi A. Comparison of the fit and fill between the Anatomic Hip femoral component and the VerSys Taper femoral component using virtual implantation on the ORTHODOC workstation. J Orthop Sci. 2003;8:352–360. [DOI] [PubMed] [Google Scholar]
- 18.Paul HA, Bargar WL, Mittlestadt B, Musits B, Taylor RH, Kazanzides P, Zuhars J, Williamson B, Hanson W. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992; 285:57–66. [PubMed] [Google Scholar]
- 19.Petis SM, Howard JL, McAuley JP, Somerville L, McCalden RW, MacDonald SJ. Comparing the long-term results of two uncemented femoral stems for total hip arthroplasty. J Arthroplasty. 2015;30:781–785. [DOI] [PubMed] [Google Scholar]
- 20.Shima Y. [Present status of surgical treatment of arthrosis deformans of the hip and summary of therapeutic evaluation] [in Japanese]. Nihon Seikeigeka Gakkai Zasshi. 1971;45:828–831. [PubMed] [Google Scholar]
- 21.Su EP, Barrack RL. Cementless femoral fixation: not all stems are created equally. Bone Joint J. 2013;95 (11 Suppl A):53–56. [DOI] [PubMed] [Google Scholar]
- 22.Yamada H, Yoshihara Y, Henmi O, Morita M, Shiromoto Y, Kawano T, Kanaji A, Ando K, Nakagawa M, Kosaki N, Fukaya E. Cementless total hip replacement: past, present, and future. J Orthop Sci. 2009;14:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]