Abstract
Background
Immediately recycling the resected bone segment in a biologic limb salvage reconstruction is an option after wide resection of bone. Intraoperative extracorporeal irradiation and freezing are the two major tumor-killing techniques applied on the fresh tumor-bearing autografts. However, graft-derived tumor recurrence and complications are concerns affecting graft survival.
Questions/Purposes
We therefore asked: (1) Is there a difference in the proportion of patients achieving union by 18 months after surgery between the groups with extracorporeal-irradiated autografts and frozen-treated autografts? (2) Is there any difference in the frequency of graft-related complications for patients receiving either an extracorporeal-irradiated or a frozen-treated autograft? (3) Is there a difference between the techniques in terms of graft-derived recurrence? (4) Are there differences in failure-free grafts, and limb and overall survivorship between autografts treated by extracorporeal irradiation or by freezing?
Methods
During the study period we treated a total of 333 patients with high-grade osteosarcoma. One hundred sixty-nine patients were excluded. Overall, 79 of the enrolled 164 patients received recycled autografts treated with extracorporeal irradiation whereas the other 85 received frozen-treated autografts. The mean followup was 82 ± 54 months for the extracorporeal irradiation group and 70 ± 25 months for the frozen autograft group, and one patient was lost to followup. Complications and graft failure (revision required for primary graft removal) were characterized by adapting the International Society of Limb Society (ISOLS) system modified for inclusion of biologic and expandable reconstruction. The primary study endpoints were the proportion of patients in each group who achieved radiographic union, and had an ISOLS grade of fair or good host graft fusion at 6, 9, 12, and 18 months after surgery. Five-year survival data for graft failure and limb amputation were analyzed by a cumulative incidence function regression model whereas the Kaplan-Meier function was used to test the 5-year overall survival rate between the two techniques.
Results
With the numbers available, no differences were found in the accumulated proportion of patients achieving union between the groups at 6, 9, 12, and 18 months. Radiographic evaluation did not show differences in the average scores of compared criteria. However in the subchondral bone subcriterion, more patients receiving frozen-treated autografts had higher scores (p = 0.03). Complications leading to a second surgery were not different between extracorporeal irradiation and frozen autografts in aspects of soft tissue failure (Type 1B), nonunion (Type 2B), structural failure (Type 3A and Type 3B), or infection (Type 4A and Type 4B). No graft-originating tumor recurrence was found and there was no difference in Type 5A tumor progression originating from soft tissue in the groups (odds ratio, 0.8; 95% CI, 0.3-2.1; p = 0.7). Neither group showed a difference in the cumulative incidence for graft failure and limb amputation. Five-year overall survival rates were 83% and 84% (p = 0.69) for extracorporeal-irradiated and frozen autografts respectively. A decrease in survivorship was seen at 50 to 100 months after surgery for the extracorporeal irradiation group.
Conclusion
We segregated the ISOLS criteria evaluating the graft-mediated tumor progression into host- or graft-derived complications (Types 5B and 5C) in this study. With the available data, there was no difference in the incidence of tumor recurrence derived from irradiation- or frozen-treated autografts. Ongoing evaluations comparing 10-year survivorship for both groups will be helpful to elucidate the possible difference found after 100 months.
Level of Evidence Level
III, therapeutic study
Introduction
The goal of surgery for primary malignant bone tumors is to remove local disease and restore limb function. Wide resection and limb salvage surgery are the standard treatments for primary malignant bone tumors such as high-grade osteosarcoma. After tumor resection, biologic and nonbiologic (tumor prostheses) techniques are available for reconstruction. Tumor prostheses are devices providing quick assembly and immediate mechanical stability. However, infection and aseptic loosening also raise concerns [2, 18]. Limb salvage with biologic reconstruction with either allografts or autografts (recycled from the resected autogenic bone segment) has advantages in host bone-graft incorporation and better longevity [7, 15, 23]. It has been reported that the overall survivorship of prostheses was 35% to71% at 10 years and 80% for irradiated autografts [10]. In addition, a tendon/ligament-bearing bone graft is preferable for soft tissue reconstruction [15] and lower risk of transmitted diseases [7]. Several methods have been developed to eradicate tumor cells before implantation, such as pasteurization [1, 4], extracorporeal irradiation [7, 17], freezing [10, 15, 19, 21], and autoclaving [22].
Currently, extracorporeal irradiation and freezing are used at our institute for treating autografts used in biologic reconstruction. Both methods aim to eradicate tumor cells from local recurrence. Compared with extracorporeal irradiation, the freezing procedure did not require an extracorporeal irradiation machine. In addition, the freezing procedure can be done in the operating room, which saves delivery time and avoids contamination. However, an intrafreezing graft fracture could compromise some advantages. Studies evaluating the use of extracorporeal irradiation [7, 9, 13, 17] or frozen autografts [10, 15, 19] and acceptable outcomes in terms of complications and graft failure-free survivorship have been reported. Owing to the unexpected events in surgical practice, we did not randomize the selection of either technique for patients receiving autografts. Up to now, a standard protocol determining the preferable pretreatment of autografts for a specific subject has not been developed. Despite observing equivalent outcomes, in our experience, irradiation and ultrafreezing exhibit effective activity in tumor eradication but jeopardize graft-host bone incorporation and graft structure [13] in different physical mechanisms. Consequently, the damaged grafts may mediate various effects on union, perioperative complications, and graft survivorship. Having science-based knowledge based on a comprehensive comparison between these two techniques would be helpful to justify a preferred technique for a specific patient.
Specifically, we asked: (1) Is there a difference in the proportion of patients achieving union by 18 months after surgery between the groups with extracorporeal-irradiated autografts and frozen-pretreated autografts? (2) Is there any difference in the frequency of graft-related complications for patients receiving either an extracorporeal-irradiated or a frozen-treated autograft? (3) Is there a difference between the techniques in terms of graft-derived recurrence? (4) Is there a difference in 5-year graft failure-free limb and overall survival rates between the autografts treated by extracorporeal irradiation or by freezing?
Methods
Patients
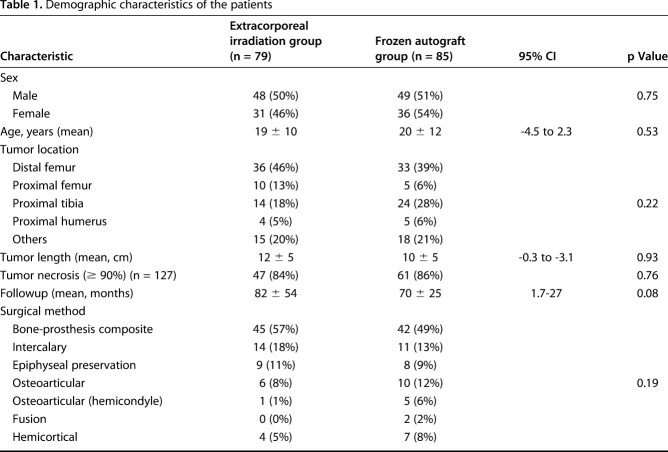
The protocol of this study was approved by the institutional review board of Taipei Veterans General Hospital (Number 2016-05-013CC). A total of 333 patients with high-grade osteosarcoma were treated at our institute between January 1998 and December 2012. One hundred sixty-nine patients were excluded owing to unplanned surgery (n = 36), loss to followup (n = 1), use of allograft (n = 91), use of tumor prosthesis (n = 22), or no reconstruction (n = 19) (Fig. 1). Finally, 164 patients with histopathologically verified high-grade osteosarcoma after wide resection and reconstruction with recycled autografts were included in the study. Before now, our institute did not have a standard protocol justifying the use of either technique. Generally, for elderly or patients with poor survival we prefer using tumor prostheses. For patients with a severe osteolytic lesion or pathologic fracture, we used allografts. Other patients undergoing reconstruction received autografts. Selection between extracorporeal irradiation and the freezing technique were determined based on the following rationales during the study period. Before October 2005 when liquid nitrogen freezing technology had not yet been implemented at our institute, extracorporeal-irradiated autografts (n = 22) were used exclusively. After October 2005, during the early stage after cryotherapy was introduced, surgeons used frozen autografts more frequently. In addition to the results showing the equivalence of outcomes with the irradiation technique, we wanted to randomize selection between the two techniques. However, some events limited complete randomization and consequently raised bias. We therefore analyzed the demographic characteristics and found no differences among the analyzed criteria in the studied population (Table 1). Seventy-nine patients received extracorporeal-irradiated autografts and 85 received frozen autografts. Of the 79 patients receiving irradiated autografts, one patient (1%) had early multiple lung metastasis and another (1%) underwent revision surgery owing to an early deep infection. One of the 85 patients in the frozen autograft group (1%) underwent an amputation owing to early local tumor recurrence. The effect of transfer bias was rare. The mean followup was 82 ± 54 months (range, 15-218 months) for the extracorporeal irradiation group and 70 ± 25 months (range, 3-123 months) for the frozen autograft group.
Fig. 1.
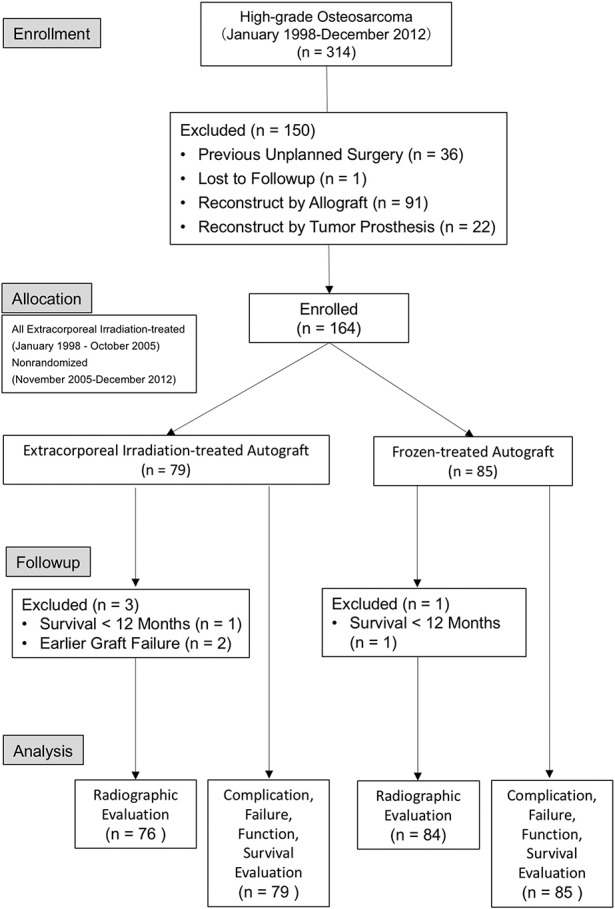
The study flow design is presented. ECIR = extracorporeal irradiation
Table 1.
Demographic characteristics of the patients
Surgical Procedures
After neoadjuvant chemotherapy, all patients received wide tumor resection and reconstruction with recycled autografts. For the extracorporeal-irradiated autografts, the bone segment was tightly wrapped with a sterile drape and sealed in doubled-layered plastic bags. The sealed bone segment was irradiated by a linear accelerator with a single dose of 150 Gy to 300 Gy. A radiation field was ensured to adequately cover the tumor-bearing bone segment. The treated bone segment was returned immediately for the reconstruction surgery.
For the frozen treatment, the resected segment was frozen in liquid nitrogen for 20 minutes, followed by slow thawing at room temperature for 15 minutes and 10-minute successive thawing in distilled water. An intrafreezing graft fracture was the major concern during the freezing procedure but could be avoided by creating tunnels through the bone medullary cavity or drilling a few holes in bone segments for homogenous distribution of liquid nitrogen. Three patients who received allografts were excluded owing to intrafreezing graft fractures during the early phase of this study. The extracorporeal-irradiated and frozen-treated autografts were reimplanted and fixed using osteosynthesis materials. For fixation, we routinely used a dual or a single plate with the cemented stem of the prosthesis to achieve rigid fixation. Unless there was poor soft tissue coverage or small bone structure, we used a single plate to fix the recycled autograft.
Rehabilitation and Followup
After surgery, patients were allowed partial weightbearing and performed muscle-strengthening exercises. Increased weightbearing and strength exercises were allowed if there was radiographic evidence of improvement in bone union. Radiographs were assessed every 4 to 6 weeks after surgery until union was achieved. MR and chest CT images were acquired quarterly during the first 2 postoperative years and every 6 months between 3 and 5 years after surgery. MR and chest CT images were evaluated annually after the 5-year followup. For patients requiring revision surgery, Type 1 complications (soft tissue failure) were managed with general wound closure, and a plastic surgeon was consulted for the flap reconstruction. For the patients showing Type 2 complications (nonunion), we generally performed surgery with secondary autograft bone grafting.
Radiographic Evaluation
Radiographic evaluation of the grafts was done using the International Society of Limb Society (ISOLS) allograft radiographic evaluation system [5, 8]. It has three specific parameters including “graft”, “bone composite” and “osteochondral”. Despite this evaluation system’s primary development for allograft transplantation, its use for evaluating autografts also has been reported [1, 16]. For the scoring system, the grades of excellent, good, fair, and poor were scored as 4, 3, 2, and 1, respectively. A senior radiologist (Y-CC) reviewed the images and evaluated the grade of union. The primary study endpoint was the proportion of patients in each group who achieved radiographic union at 6, 9, 12, and 18 months.
We defined bone “union” as being achieved when the radiographic image showed the graft-host bone junction fusion exceeded more than 75% of the cortical thickness (corresponding to “good fusion” in the ISOLS system) or the fusion was graded as “fair” (25%-75% fusion) without further improvement. Fusions that failed to meet one of the criteria of “union” were considered “nonunions”. For grafts with more than one graft-host bone junction (intercalary, epiphyseal preservation, hemicondylar, hemicortical, or fusion surgeries), union was determined by all junctions achieving union.
Modification on the Characterization of Autograft-derived Complications and Failures
For a transplanted tumor-bearing autograft, residual tumor cells may lead to tumor recurrence or progression which can impair rehabilitation. The current version of the ISOLS classification system characterizes two types of tumor progression: “Type 5A - soft tissue tumor progression with allograft contamination”, and “Type 5B - bony tissue tumor progression with allograft contamination” (see Appendix, Supplemental Digital Content 1) [8]. To compare the tumor-killing effectiveness between the two techniques, we need to further specifically identify the graft-originated tumor recurrence from the host bone-derived recurrences. Therefore we modified the current ISOLS classification system addressing the tumor progression. First, we amended Subtype 5C specifying secondary tumor recurrence originating from the implanted grafts that were not characterized in the ISOLS classification. The graft-originated tumor recurrence was characterized as graft invasion with recurrent tumor observed on MR images or with pathology verification. If the MR image showed soft tissue tumor recurrence without bone involvement, it was characterized as Type 5A. For a recurrent tumor observed on an MR image as being close to a recycled autograft, the recurrence was classified as Type 5C if the pathology results revealed graft invasion, indicating an autograft-originated tumor recurrence. Subtype 5B specifically indicated host bony tissue-derived tumor progression. In addition, Type 6 failures in pediatric patients were not applicable in this study. Second, we determined the “complications” as those that could be managed without removal of entire grafts. Complications resulting in revision surgery to remove entire grafts were characterized as “failures”.
Statistics
Cumulative incidence function for graft failure and limb amputation was determined using the cmprsk package (Version 2.2-7) from the R program (R Development Core Team 2010, R Foundation for Statistical Computing, Vienna, Austria). Overall survival rate was calculated and compared using the Kaplan-Meier method. Categorical variables were compared using a chi-square test, whereas continuous variables were analyzed using a t test. All analyses were conducted using SPSS Version 24.0 (IBM Corporation, Armonk, NY, USA), and a two-sided probability less than 0.05 was considered statistically significant. Post hoc analysis was used to examine the power of the statistical results.
Results
Proportion of Patients Achieving Union at Four Intervals During 18 Months
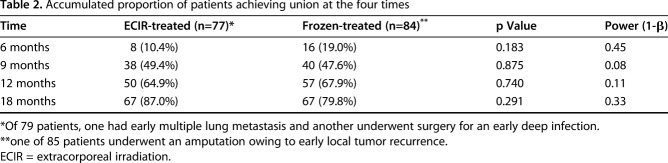
In both groups, most patients achieved postsurgical union by 20 months (Fig. 2). At the end of 6 months after surgery, the union rate for the patients receiving extracorporeal-irradiated autografts was 10% (eight of 77), and it was 19% (16 of 84) for the group receiving frozen autografts (Table 2). At 9 months, 49% (38 of 77) of patients who received irradiation treatment achieved union whereas the ratio for patients receiving the frozen treatment was 48% (40 of 84). At 12 months, the accumulated ratios of patients achieving union were 65% (50 of 77) and 68% (57 of 84) for the patients receiving irradiation and frozen treatment respectively. After 18 months postoperative, 87% (67 of 77) of patients receiving extracorporeal irradiation achieved union whereas the rate was 80% (67 of 84) for the patients receiving frozen autografts. With the numbers available, we found no difference between the irradiated and frozen autografts regarding the union rate at the four interval evaluations, and no difference was found in the union rate between the patients in either group at the four times. However, the results of post hoc analysis showed a power smaller than 0.8, indicating that the nondifference needs to be further verified with a larger population.
Fig. 2 A-B.
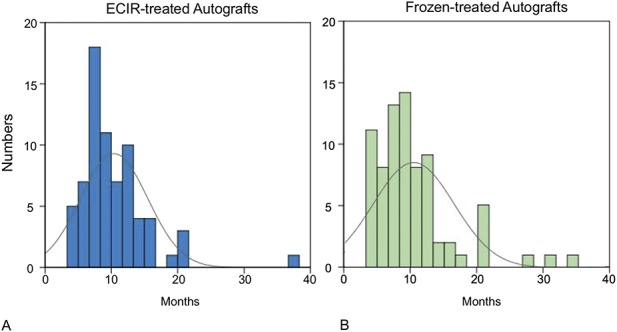
The graph shows the chronologic distribution for the numbers of unions in the (A) extracorporeal irradiation- (ECIR) and (B) frozen-treated allograft groups. ECIR = extracorporeal irradiation.
Table 2.
Accumulated proportion of patients achieving union at the four times
Graft-related Complications and Failure
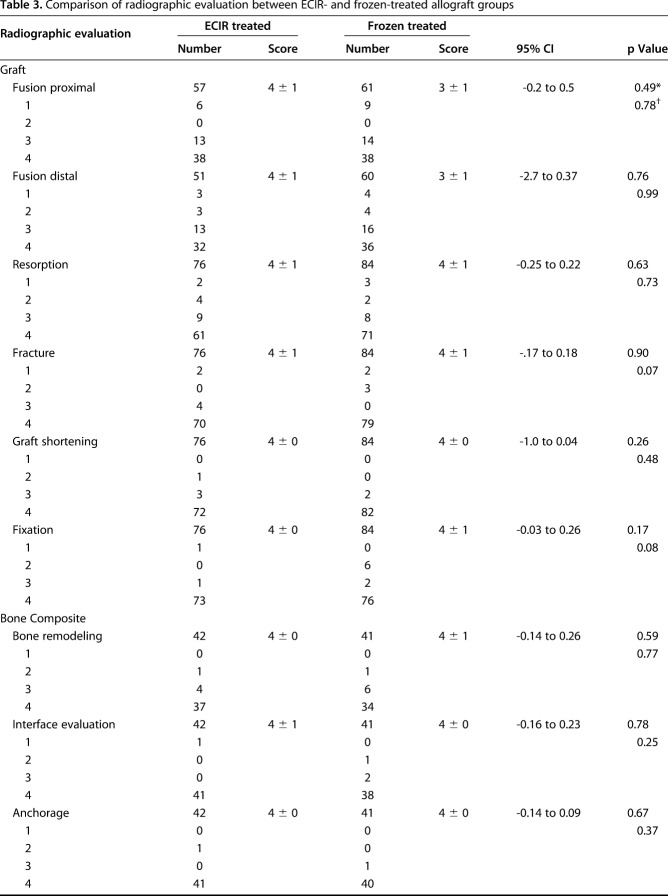
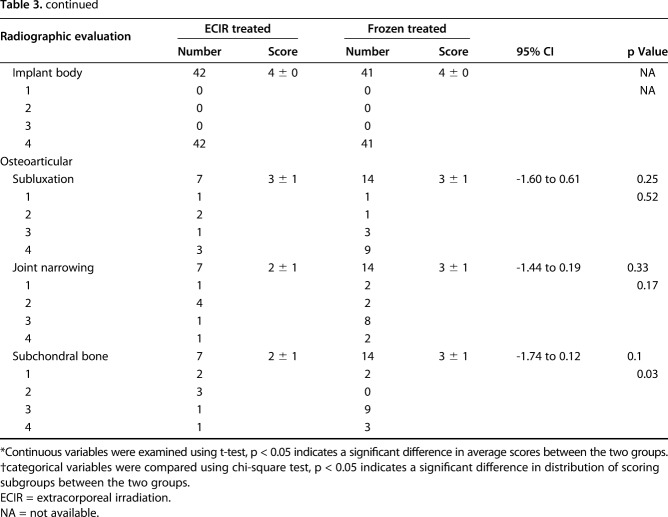
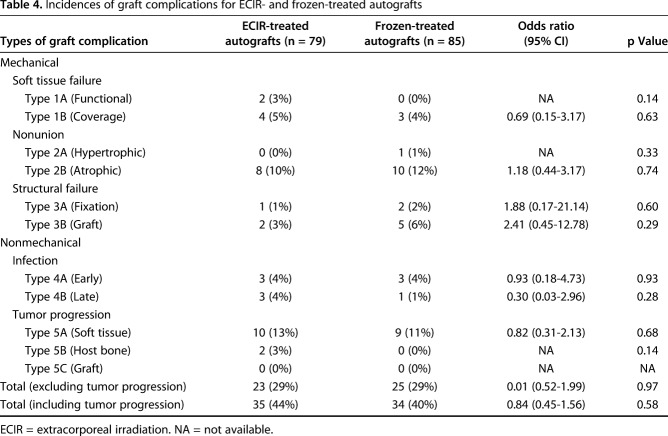
With the results of radiographic evaluation on the “graft” and “bone composite”-specific parameters, we did not find differences between the extracorporeal-irradiated and frozen-treated autografts in terms of mean scores for graft fusion, resorption, fracture, shortening, and fixation (Table 3). With further analysis of the scoring categories, we found that subchondral bone was graded with higher scores for the patients receiving frozen autografts (p = 0.03) (Table 3).
Table 3.
Comparison of radiographic evaluation between ECIR- and frozen-treated allograft groups
Among 19 patients with graft nonunion, 18 were classified as having an atrophic subtype. We did not find a difference in the incidence of atrophic nonunion between the groups receiving irradiated (10%, eight of 79) or frozen (12%, 10 of 85) autografts (odds ratio [OR], 1.18; 95% CI, 0.44-3.17; p = 0.74) (Table 4). The complication rates attributable to structure failure (Types 3A and B) and infection (Types 4A and B) also were comparable (Table 4). If tumor progression (Types 5A, B, and C) was excluded, the total numbers of complications were 23 (29%) and 25(29%) for the extracorporeal-irradiated and frozen-treated autograft groups, respectively (OR, 0.01; 95% CI, 0.51-1.99; p = 0.97). Most complications (87% in extracorporeal irradiation-treated group and 88% in frozen-treated group) occurred during the first 3 years after surgery (Fig. 3). The complication rates were decreased (11% in extracorporeal irradiation- and 4% in frozen-treated groups) by the end of the 5-year followup.
Table 4.
Incidences of graft complications for ECIR- and frozen-treated autografts
Fig. 3 A-B.
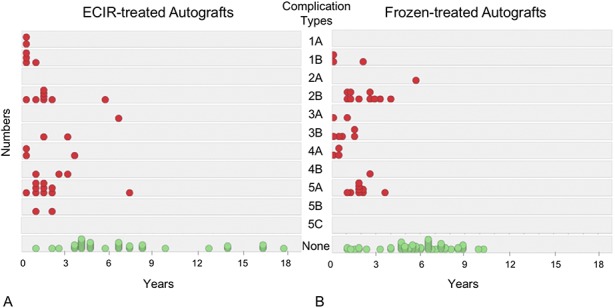
The graphs show the chronologic distribution for the numbers and subtypes of graft complications in the (A) extracorporeal irradiation- (ECIR) and (B) frozen-treated allograft groups.
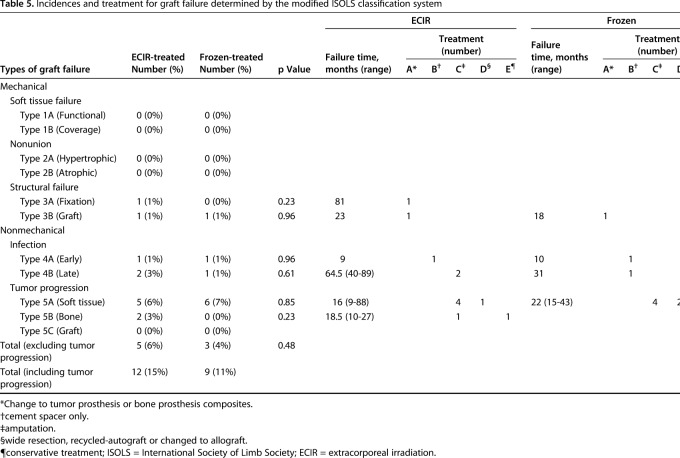
We further characterized “graft failure” as the events resulting in secondary surgery to remove the primary grafts and to implement alternative management (Table 5). If the patients with tumor progression were excluded, 6% (five of 79) of the patients with complications in the extracorporeal irradiation-treatment group and 4% (three of 85) in the frozen-treatment group had progression of their graft failures (Table 5). All Types 1 and 2 complications were successfully treated with additional procedures without graft failure (Table 5).
Table 5.
Incidences and treatment for graft failure determined by the modified ISOLS classification system
Graft-derived Tumor Progression
Tumor progression occurred in 21 patients but no tumors originated from the transplanted autografts (Type 5C, Table 4). The overall tumor recurrence rate was 15% (12 of 79) in the extracorporeal irradiation-treated group and 11% (nine of 85) for the frozen-treated group, and the available results did not show a difference between the two groups (OR, 0.66; 95% CI, 0.26-1.67; p = 0.37). Tumor progression originated mainly from soft tissue (13%, 10 of 79 for irradiation and 11%, nine of 85 for frozen-treated [Type 5A]) (Table 4) rather than host bone (3%, two of 79 for irradiation and none for frozen-treated [Type 5B]) (Table 4).
Among the patients with tumor recurrence, 62% (13 of 21) had progression of graft failure that required graft removal. Seven of the 13 tumor recurrence related-graft failures were in the irradiation-treated group whereas the other six were in the frozen-treated group (Table 5).
Failure-free Graft, Limb and Overall Survivorship
With the available data, irradiation and freezing showed no differences in the probability of graft failure (p = 0.36, Fig. 4A) or limb amputation (p = 0.81, Fig. 4B). The 5-year overall survival rates were 83% for extracorporeal-irradiated and 84% for frozen-treated autografts (p = 0.69, Fig. 4C). A reduction in the overall survival rates was found at approximately 100 months for the extracorporeal irradiation group.
Fig. 4 A-C.
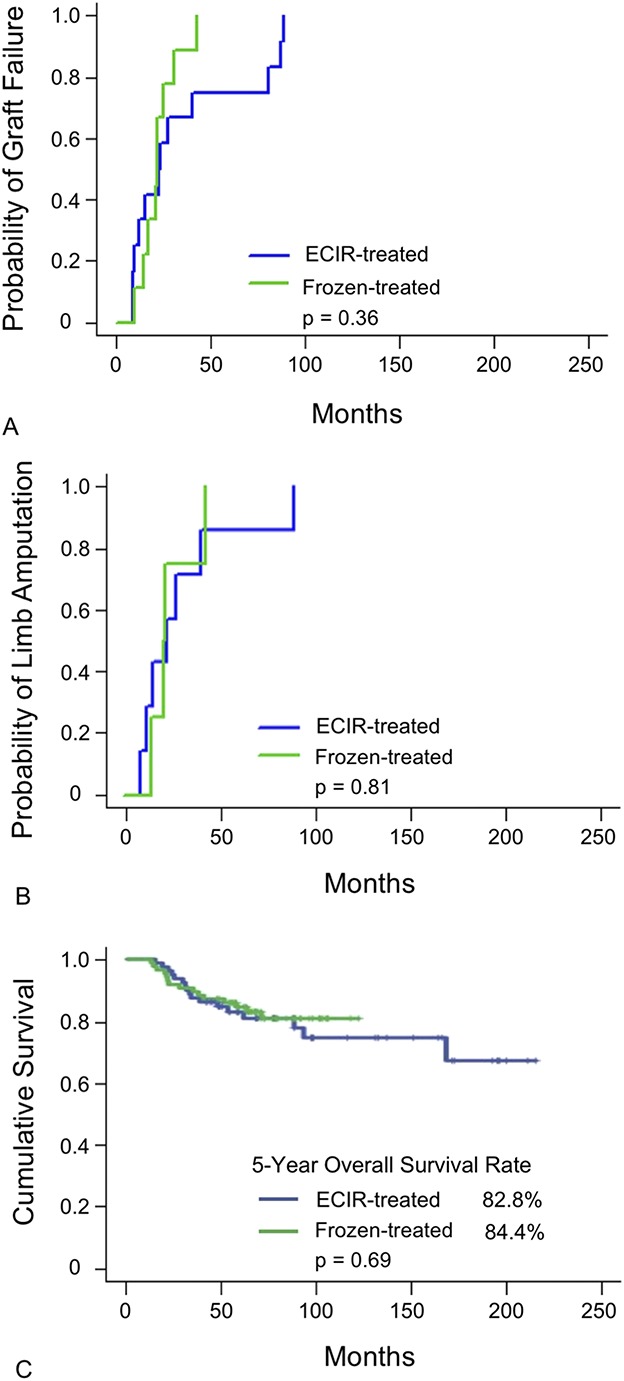
The results of survivorship modeling are shown for (A) cumulative incidence function modeling for graft failure, (B) cumulative incidence function modeling for limb amputation, and (C) Kaplan-Meier modeling for 5-year overall survival rate. ECIR = extracorporeal irradiation.
Discussion
Recycled tumor-bearing autografts are an alternative option if prosthetic devices or allografts are not available owing to the cost or lack of availability from the bone bank. Tumor recurrence usually leading to graft failure is a major concern for use of autografts, despite that studies on small series using either allografts or recycled autografts have shown favorable bone union and enhanced sustainable longevity [7, 23]. Extracorporeal irradiation and freezing are two techniques commonly used for treating autografts to eliminate residual tumor cells. Some studies have shown acceptable survival rates and function [10, 15-17]. However, the effectiveness of tumor eradication for both techniques has not yet been fully determined. In addition, complications such as graft fracture attributable to irradiation or shock from freezing and thawing, and infection derived from an autograft’s exposure to an ambient condition, may negatively affect bone-graft union and graft survivorship. Despite that we observed equivalent outcomes in this study, comprehensive evaluation of the clinical outcomes using a generally accepted standard protocol of these two techniques is required to justify either technique as being suitable for a specific patient. In this study, we found no difference in union rates between irradiation and frozen treatment on the recycled autografts by 18 months. We also did not find differences in the incidences of complications and graft failure.
This study has several limitations. Owing to later implementation of the frozen technique at our institution, scientific rationales for determining the selection of one technique over another for individual patients were not considered. Selection bias subject to surgical methods may affect the clinical outcomes. For example, Hayashi et al. [7] reported that irradiated osteoarticular bone grafts were not favorable for weightbearing joints owing to a high incidence of reoperation (47%). Igarashi et al. [10] reported that for patients receiving liquid nitrogen frozen-osteoarticular autografts, a high incidence (44%) of graft failure was attributable to fracture or infection, however all composite and intercalary grafts survived. In our study, demographic analysis that showed no difference in terms of surgical methods supported the decreased selection bias-derived effect. Later implementation of the freezing technique also led to a shorter mean observation period that was close to the margin indicating a difference. The modeling curve for the three survival rates consistently appeared as an apparent reduction at approximately 100 months for the patients receiving irradiated autografts but it likely was absent in the frozen-treated group. In this study we analyzed only the 5-year failure-free grafts and limb and overall survivorship but not the 10-year survivorship owing to a shorter mean followup for the frozen-treated group. Therefore we are not able to conclude survivorship longer than 5 years between these two groups. In addition, there are other variable factors such as oncogenic structure damage, quality of grafts, sites of reconstruction, and stabilization during reconstruction that may affect the generalization of this study. All these related factors need to be considered and included in the study design for a future investigation.
In both groups, more than 80% of patients achieved union by 18 months after surgery, with no difference in terms of the accumulated union rate at four times. Most unions were detected within 8 to 14 months after surgery. Fourteen grafts united late (> 18 months) without additional surgery. A long-term evaluation by Igarashi et al. [10] of the clinical outcomes of 36 patients receiving frozen autografts revealed a union rate of 72% (26 of 36), which was close to our result. In our study the nonunion rate was 10% for patients receiving extracorporeal irradiation and frozen treatment. In another long-term study of irradiation treatment, it was 16% (12 of 74) [7]. Longer union time (16 months) [4, 14] and higher nonunion rates (20% to 33%) [4, 11] have been reported for reconstruction using pasteurized autografts. In an 11-patient study, shorter union time was reported for frozen-treated autografts compared with pasteurization treatment (7 months versus 11 months) [14]. Better union associated with frozen autografts rather than hyperthermic pasteurization may be attributed to preservation of active BMP-7 [20] that is able to induce new bone formation in vivo. Therefore we theorize that irradiation and freezing procedures could preserve more active growth factors required for osteogenesis such as BMPs and VEGF. A previous histologic analysis involving patients receiving retrieval surgery showed osteonecrosis in subchondral bones, suggesting that subchondral bone is vulnerable to irradiation exposure [6]. Intriguingly, frozen-treated autografts exhibited a trend associated with higher-scoring categories in subchondral bone performance in our study. However, because of the small number of patients receiving osteoarticular bone grafts, the validity of the difference needs to be further verified. We found fair performance for extracorporeal-irradiated and frozen-treated autografts for all osteoarticular-specific criteria including subluxation, joint narrowing, and subchondral bone. Excellent radiographic outcomes were found for evaluation of bone prosthesis composites and there was no difference between extracorporeal-irradiated and frozen autografts. These results are consistent with results from prior studies [3, 23].
The total complication rate was 44% (35 of 79) for irradiation treatment in our study, and Hayashi et al. [7], in a long-term study, reported a complication incidence of 51% (38 of 74). For the patients receiving frozen autografts, we found a complication rate of 40% (34 of 85) that was comparable with a previously reported 39% rate (14 of 36) [10]. Most complications (approximately 90%) in both groups occurred during the first 3 years after surgery, after which they were found less frequently. In the current study, we specifically defined “graft failure” as those situations in which the graft subsequently was surgically removed. Hayashi et al. [7] reported a complication-driven graft failure rate of 11% (eight of 74) for patients receiving irradiated autografts; it was 15% in our study. In general, the overall rates of complications and graft failure obtained in our study were similar to those of Hayashi et al. [7]. Specifically, infection occurred in 8% (six of 79) of patients in the irradiation group in our study and in 18% (13 of 74) of patients in the study by Hayashi et al. [7]. Infection occurred in 5% (four of 85) of our patients receiving frozen autografts, whereas it was 11% in the study by Igarashi et al. [10].
Use of tumor-bearing bone grafts raised concerns regarding tumor recurrence. To compare the tumor-eradicating effect between irradiation and freezing, a modified ISOLS classification system of allograft failures was adapted by amending a subtype complication, Type 5C, specifying tumor recurrence or progression originating from the implanted autografts. Our results are consistent with those of a previous study [12] showing the incidence of tumor recurrence in a range of 7% to 20%. In our study however, no tumor recurrence was derived from extracorporeal-irradiated or frozen-treated autografts (Type 5C). This indicates that either 150 Gy to 300 Gy irradiation or 20 minutes of liquid nitrogen freezing and slow thawing achieve similar and reliable efficacy in eradicating tumor cells.
Regarding survivorship, Hayashi et al. [7] reported a reduction in disease-specific and irradiated bone graft survival rates for 100 months after surgery. Igarashi et al. [10] also reported a dramatic decrease in the graft survival rate by 100 months after surgery among a small series of patients receiving frozen osteoarticular bone grafts, but there was no difference in survival rate for reconstructions receiving composites and intercalary grafts in their study. It is an intriguing issue and deserves further investigation.
Based on the current study, a protocol is needed to better control our choice of surgical method and autograft treatment. We will continue to collect results from our patients for a 10-year survivorship analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that may pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Taipei Veterans General Hospital approved the human protocol for this investigation, and each author certifies that all investigations were conducted in conformity with ethical principles of research.
This study was done at Taipei Veterans General Hospital, Taipei, Taiwan.
References
- 1.Ahmed AR, Manabe J, Kawaguchi N, Matsumoto S, Matsushita Y. Radiographic analysis of pasteurized autologous bone graft. Skeletal Radiol. 2003;32:454–461. [DOI] [PubMed] [Google Scholar]
- 2.Capanna R, Scoccianti G, Frenos F, Vilardi A, Beltrami G, Campanacci DA. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Relat Res. 2015;473:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WM, Chen TH, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg Br. 2002;84:1156–1161. [DOI] [PubMed] [Google Scholar]
- 4.Eid AS, Jeon DG, Song WS, Lee SY, Cho WH. Pasteurized autograft-prosthesis composite for proximal femoral reconstruction: an alternative to allograft composite. Arch Orthop Trauma Surg. 2011;131:729–737. [DOI] [PubMed] [Google Scholar]
- 5.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 6.Hatano H, Ogose A, Hotta T, Endo N, Umezu H, Morita T. Extracorporeal irradiated autogenous osteochondral graft: a histological study. J Bone Joint Surg Br. 2005;87:1006–1011. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Araki N, Koizumi M, Suzuki O, Seo Y, Naka N, Isohashi F, Myoui A, Yoshioka Y, Teshima T, Ueda T, Yoshikawa H, Ogawa K. Long-term results of intraoperative extracorporeal irradiation of autogenous bone grafts on primary bone and soft tissue malignancies. Acta Oncol. 2015;54:138–141. [DOI] [PubMed] [Google Scholar]
- 8.Henderson ER, O'Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system including biological and expandable reconstructions. Bone Joint J. 2014;96:1436–1440. [DOI] [PubMed] [Google Scholar]
- 9.Hong AM, Millington S, Ahern V, McCowage G, Boyle R, Tattersall M, Haydu L, Stalley PD. Limb preservation surgery with extracorporeal irradiation in the management of malignant bone tumor: the oncological outcomes of 101 patients. Ann Oncol. 2013;24:2676–2680. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi K, Yamamoto N, Shirai T, Hayashi K, Nishida H, Kimura H, Takeuchi A, Tsuchiya H. The long-term outcome following the use of frozen autograft treated with liquid nitrogen in the management of bone and soft-tissue sarcomas. Bone Joint J. 2014;96:555–561. [DOI] [PubMed] [Google Scholar]
- 11.Manabe J, Ahmed AR, Kawaguchi N, Matsumoto S, Kuroda H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:258–266. [DOI] [PubMed] [Google Scholar]
- 12.Mottard S, Grimer RJ, Abudu A, Carter SR, Tillman RM, Jeys L, Spooner D. Biological reconstruction after excision, irradiation and reimplantation of diaphyseal tibial tumours using an ipsilateral vascularised fibular graft. J Bone Joint Surg Br. 2012;94:1282–1287. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Abudu A, Grimer RJ, Carter SR, Jeys L, Tillman RM. The clinical outcomes of extracorporeal irradiated and re-implanted cemented autologous bone graft of femoral diaphysis after tumour resection. Int Orthop. 2013;37:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura K, Miyamoto S, Sakuraba M, Fujiwara T, Chuman H, Kawai A. Intercalary reconstruction after wide resection of malignant bone tumors of the lower extremity using a composite graft with a devitalized autograft and a vascularized fibula. Sarcoma. 2015;2015:861575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paholpak P, Sirichativapee W, Wisanuyotin T, Kosuwon W, Jeeravipoolvarn P. Clinical results of primary malignant musculoskeletal tumor treated by wide resection and recycling autograft reconstruction using liquid nitrogen. Asia Pac J Clin Oncol. 2015;11:114–120. [DOI] [PubMed] [Google Scholar]
- 16.Poffyn B, Sys G, Van Maele G, Van Hoorebeke L, Forsyth R, Verstraete K, Uyttendaele D. Radiographic analysis of extracorporeally irradiated autografts. Skeletal Radiol. 2010;39:999–1008. [DOI] [PubMed] [Google Scholar]
- 17.Sharma DN, Rastogi S, Bakhshi S, Rath GK, Julka PK, Laviraj MA, Khan SA, Kumar A. Role of extracorporeal irradiation in malignant bone tumors. Indian J Cancer. 2013;50:306–309. [DOI] [PubMed] [Google Scholar]
- 18.Shehadeh A, Noveau J, Malawer M, Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010;468:2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subhadrabandhu S, Takeuchi A, Yamamoto N, Shirai T, Nishida H, Hayashi K, Miwa S, Tsuchiya H. Frozen autograft-prosthesis composite reconstruction in malignant bone tumors. Orthopedics. 2015;38:e911–918. [DOI] [PubMed] [Google Scholar]
- 20.Takata M, Sugimoto N, Yamamoto N, Shirai T, Hayashi K, Nishida H, Tanzawa Y, Kimura H, Miwa S, Takeuchi A, Tsuchiya H. Activity of bone morphogenetic protein-7 after treatment at various temperatures: freezing vs. pasteurization vs. allograft. Cryobiology. 2011;63:235–239. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya H, Wan SL, Sakayama K, Yamamoto N, Nishida H, Tomita K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J Bone Joint Surg Br. 2005;87:218–225. [DOI] [PubMed] [Google Scholar]
- 22.Umer M, Umer HM, Qadir I, Rashid H, Awan R, Askari R, Ashraf S. Autoclaved tumor bone for skeletal reconstruction in paediatric patients: a low cost alternative in developing countries. Biomed Res Int. 2013;2013:698461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CS, Wu PK, Chen CF, Chen WM, Liu CL, Chen TH. Bone-prosthesis composite with rotating hinged-knee prosthesis in limb salvage surgery for high-grade sarcoma around the knee. J Arthroplasty. 2015;30:90–94. [DOI] [PubMed] [Google Scholar]