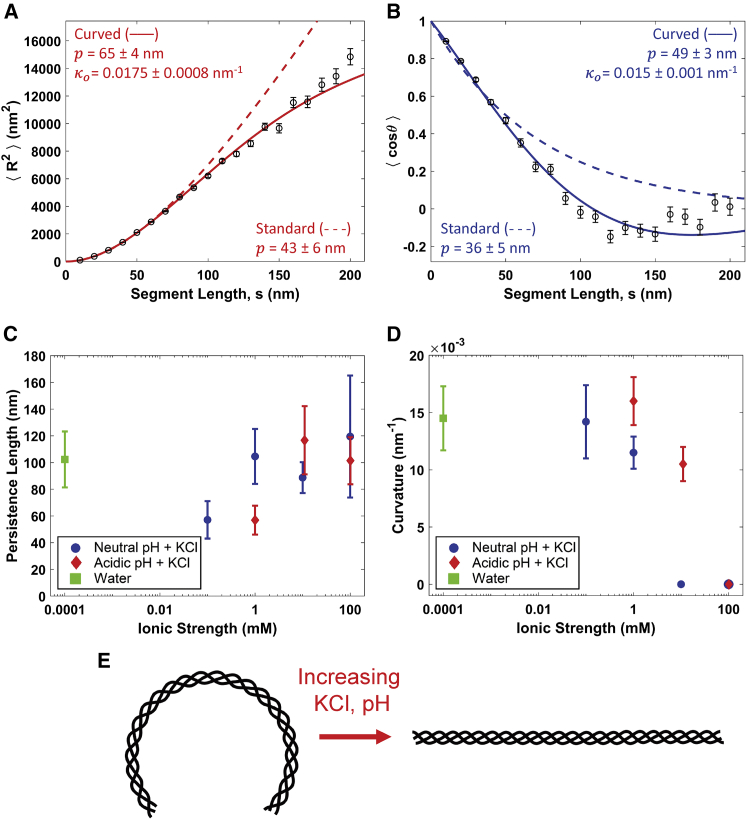
Figure 4.
At low ionic strength, collagen is better described as a cWLC. (A) Fits of the mean-square end-to-end distance using both the standard (Eq. 1, dashed red line) and curved (Eq. 3, solid red line) WLC models to data extracted from rat tail type I collagen deposited from 1 mM HCl. (B) Corresponding fits of the standard (Eq. 2, dashed blue line) and curved (Eq. 4, solid blue line) WLC tangent vector correlation functions. The data are more accurately described by the curved model equations, as shown statistically by a significant improvement in reduced χ2 values (see text). The standard WLC underestimates the persistence length of the collagens in this condition, presumably interpreting the induced curvature as additional fluctuation. (C) Persistence lengths for the different cosolute conditions presented in Fig. 3 Rather than the trend with ionic strength seen with the standard WLC fits, there is a less significant and less obviously monotonic variation in persistence length as a function of ionic strength. Instead, the observed systematic conformational changes are attributed to a variation in innate curvature, which drops as the ionic strength of the solution is increased (D). For the purposes of graphical representation, water was assumed to have an ionic strength of 10−7 M. Error bars on and values are as described for Fig. 2, and error bars on p and κo are obtained as described for Fig. 3. (E) A schematic illustrating the transition of collagen from a molecule with a curved backbone at low ionic strength and pH ∼3 (left) to one with a straight backbone at higher ionic strength and neutral pH (right). The persistence length characterizes fluctuations about this lowest-energy conformation. To view this figure in color, go online.