Abstract
Introduction
The cerebrospinal fluid neurogranin (Ng)/β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) ratio may reflect synaptic affection resulting from reduced beta-amyloid (Aβ) clearance. We hypothesize that increased Ng/BACE1 ratio predicts the earliest cognitive decline in Alzheimer's disease.
Methods
We compared Ng/BACE1 levels between cases with subjective cognitive decline (n = 18) and mild cognitive impairment (n = 20) both with amyloid plaques and healthy controls (APOE-ε4+, n = 16; APOE-ε4-, n = 20). We performed regression analyses between cerebrospinal fluid levels, baseline hippocampal and amygdala volumes, and pertinent cognitive measures (memory, attention, Mini Mental State Examination [MMSE]) at baseline and after 2 years.
Results
Ng/BACE1 levels were elevated in both subjective cognitive decline and mild cognitive impairment compared to healthy controls. Higher Ng/BACE1 ratio was associated with lower hippocampal and amygdala volumes; lower baseline memory functions, attention, and MMSE; and significant decline in MMSE and memory function at 2-year follow-up.
Discussion
High Ng/BACE1 ratio predicts cognitive decline also in preclinical cases with amyloid plaques.
Keywords: Alzheimer's disease, MCI (mild cognitive impairment), SCD (subjective cognitive decline), MRI, Memory, Cognition, Synaptic loss, Cerebrospinal fluid (CSF), CSF neurogranin, CSF BACE1
Highlights
-
•
CSF Ng/BACE1 may confer synapse loss tied to β-amyloid disease mechanism.
-
•
CSF Ng/BACE1 is increased already in preclinical cases with amyloid plaques
-
•
High CSF Ng/BACE1 is the only biomarker related to lower baseline memory function.
-
•
High CSF Ng is the only biomarker related to reduced baseline hippocampal volume.
-
•
High CSF Ng predicts decline in verbal memory function at 2-year follow-up.
1. Introduction
In Alzheimer's disease (AD), amyloid-β precursor protein (AβPP) metabolizes to Aβ-peptide, which precipitates in amyloid plaques [1]. Increased CSF neurogranin is related to synaptic loss, cognitive decline, and reductions in hippocampal volume in mild cognitive impairment (MCI) and dementia due to AD. Moreover, increased CSF neurogranin may distinguish AD from other neurodegenerative diseases [2], [3], [4], [5]. Previously, we showed an inverse relationship between CSF neurogranin and the CSF Aβ1–42/Aβ1–40 ratio in MCI and dementia, suggesting that synaptic loss and AβPP metabolism may be linked [6]. Neurogranin is highly expressed in dendritic spines in hippocampal and amygdalar pyramidal cells and is linked to postsynaptic signal transduction [7], [8]. The β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is linked to presynaptic AβPP metabolism [9], [10]. Aβ-oligomers accumulate at synaptic terminals and may disrupt pyramidal cell N-methyl-D-aspartate (NMDA) receptors and postsynaptic Ca2+ homeostasis [11], [12], [13], putatively leading to synapse loss. The APOE-ε4 allele is a major genetic risk factor for AD and may enhance synaptotoxic oligomerization of Aβ-peptides [11], [14], [15].
As BACE1 is a rate-limiting step in the production of Aβ species [9], [10], inhibitors are tested [16]. Clinical and biomarker studies in AD cases have shown contradictory results [17], [18]. CSF Aβ1–42, as a marker for amyloid plaques (A), and CSF phosphorylated and CSF total tau, as markers for neurofibrillary tangles (T) and neurodegeneration (N), have been combined to the A/T/N stage marker for AD [19]. BACE1 levels have been shown to correlate with markers of neuronal degradation and neurofibrillary tangles (total and phosphorylated tau) [20], as well as synaptic loss (neurogranin), but not with Aβ [21], suggesting a relationship to neurodegeneration. Associated biomarkers can be explored as ratios, which, in some cases, have shown to offer better diagnostic performance, for example, the CSF Aβ1–42/Aβ1–40 ratio [22]. Recently, we compared several CSF measures as single analytes and ratios to cognitive decline and found that an increased ratio between CSF neurogranin trunc P75 and BACE1 (Ng/BACE1) was the only robust correlate of cognitive decline in MCI cases due to AD [21]. We propose that this ratio could sensitively reflect early synapse affection in AD linked to accumulation of toxic Aβ-oligomers at synaptic terminals.
Thus, we hypothesize that increased Ng/BACE1 ratio may herald development of cognitive deficits at a preclinical stage of AD [23], [24]. To test this hypothesis, we included cases early in the AD trajectory (i.e., cases with subjective cognitive decline (SCD) and MCI with amyloid plaques) [19], [25] and healthy APOE-ɛ4+ and APOE-ɛ4- control groups. We compared levels of Ng/BACE1 between the groups, relate Ng/BACE1 to AD biomarker severity using the A/T/N classification scheme [19], and explore relationships to baseline hippocampal and amygdala volumes and cognitive decline at 2-year follow-up.
2. Methods and materials
2.1. The Dementia Disease Initiation cohort
This study was a part of the Norwegian multicenter study, “Dementia Disease Initiation” (DDI) [26]. DDI uses a standardized protocol for participant selection, assessment, and disease-stage classification (SCD, MCI, and dementia) according to published criteria [25], [27], [28]. Participants were recruited from referrals to local memory clinics or self-referrals responding to advertisements in media, newspapers, or news bulletins. Healthy controls were recruited from spouses of participants with either MCI or SCD, volunteers responding to media advertisements or news bulletins, and from cognitively healthy patients who completed lumbar puncture for orthopedic surgery. Criteria for inclusion were age between 40 and 80 years and a native language of Norwegian, Swedish, or Danish. Exclusion criteria were brain trauma or disorder, including clinical stroke, dementia, severe psychiatric disorder, severe somatic disease that might influence the cognitive functions, intellectual disability, or other developmental disorders. The cohort described here was recruited between 2013 and 2017. For further description of the DDI cohort and methods, refer to the study by Fladby et al. (2017) [26]. Participants were assessed at baseline, and a subset had come to 2-year follow-up examination.
2.2. CSF collection and handling
Procedures were as described previously [26]. All CSF samples were analyzed at the Department of Interdisciplinary Laboratory Medicine and Medical Biochemistry at Akershus University Hospital, and samples from all other sites were frozen before sending to this laboratory following BIOMARKAPD SOPs as also described previously [29].
2.3. Protein biomarker measurements
Commercial enzyme-linked immunosorbent assays based on monoclonal antibodies were used to measure CSF levels of the following protein biomarkers: Aβ1–42, t-tau, and p-tau were determined using Innotest Aβ (1–42), Innotest h-Tau Ag, and Innotest Phospho-Tau (181P) (Fujirebio, Ghent, Belgium), respectively. BACE1 and neurogranin (trunc P75) levels were determined using kits from EUROIMMUN AG (Lübeck, Germany) as described in detail elsewhere [21]. All samples were analyzed in duplicates and reanalyzed if relative deviations (RDs) exceeded 20% and quality control samples with RD threshold of 15% controlled for interplate and interday variation.
2.4. Participant selection, study design, and A/T/N classification
For the purposes of the present study, we selected participants from the DDI cohort to construct four groups according to the study design criteria: (1) healthy controls with low risk of AD (n = 20, APOE-ɛ4-); (2) healthy controls with increased risk of AD (at least one APOE-ɛ4 allele and first degree relative with dementia, n = 16, APOE-ɛ4+); (3) SCD (n = 18) with CSF confirmed amyloid pathology; and (4) MCI (n = 20) with CSF confirmed amyloid pathology. In addition, participants were classified according to the A/T/N classification scheme for AD using CSF biomarkers [19]. A+ denotes (CSF amyloid pathology only), A + N+ (CSF amyloid pathology and neurodegenerative marker), and A + N + T+ (CSF amyloid pathology, neurodegenerative marker, and marker of neurofibrillary tangles). The following cutoff values for CSF total tau (t-tau) and phosphorylated tau (p-tau) abnormality were applied according to the laboratory recommendations (modified from the study by Sjögren et al. [30]); t-tau is >300 pg/mL for age <50 years, >450 pg/mL for age 50–69 years, and >500 pg/mL for age ≥70 years and p-tau ≥80 pg/mL. An optimal cutoff at CSF Aβ1–42 < 708 for amyloid plaque pathology was determined following DDI PET [18F]-flutemetamol uptake studies [31]. Amyloid-positive cases were screened in accordance with the A/T/N classification scheme [19] before inclusion to ensure equal distribution of pathological markers between SCD and MCI groups. For demographics and study cohort characteristics, please see Table 1.
Table 1.
Between-group comparisons between demographics, cognitive, AD, and A/T/N biomarker characteristics and APOE-ε4+/− distribution
| Variable | Groups |
F/χ2 and ηp2/η2(P) | ANOVA contrasts (P)/Dunn's pairwise comparisons |
||||||
|---|---|---|---|---|---|---|---|---|---|
| APOE-ε4− controls (n = 20) | APOE-ε4+ controls (n = 16) | Aβ+ SCD (n = 18) | Aβ+ MCI (n = 20) | 1 vs 2 | 3 vs 1 and 2 | 4 vs 1 and 2 | 3 vs 4 | ||
| Age mean (SD) | 62.8 (9.6) | 59.1 (8.5) | 66.7 (6.8) | 66.8 (7.4) | F = 4.3, ηp2 = .14 (<.01) | n.s. | <.05 | <.01 | n.s |
| Female, n (%) | 10 (50%) | 9 (56%) | 8 (44%) | 12 (57%) | χ2 = 0.8, η2 = .23 (n.s.) | ∗ | ∗ | ∗ | ∗ |
| MMSE mean (SD) | 29.4 (0.7) | 29.5 (0.7) | 29.2 (0.8) | 26.9 (2.2) | χ2 = 19.4 (<.0001) | n.s. | n.s. | <.001 | <.01 |
| CERAD learning T-score mean (SD) | 47.8 (10.8) | 54.1 (10.7) | 49.6 (8.2) | 36.3 (10.3) | F = 10.1, ηp2 = .31(<.001) | n.s. | n.s. | <.001 | <.001 |
| CERAD recall T-score mean (SD) | 45.1 (13.3) | 55.0 (6.1) | 50.4 (10.0) | 35.1 (10.5) | χ2 = 25.2, η2 = .32 (<.001) | n.s. | n.s. | <.001 | <.001 |
| TMT-A T-score mean (SD) | 50.2 (10.5) | 49.3 (7.8) | 50.3 (6.4) | 41.0 (6.7) | F = 6.2, ηp2 = .22 (<.001) | n.s. | n.s. | <.001 | <.01 |
| TMT-B T-score mean (SD) | 54.2 (7.2) | 52.0 (9.5) | 48.7 (7.9) | 39.5 (9.7) | F = 10.3, ηp2 = .32 (<.001) | n.s. | n.s. | <.001 | <.05 |
| CSF Aβ1–42 mean (SD) | 1082 (188) | 996 (175) | 530 (98) | 496 (117) | χ2 = 56.2, η2 = .76 (<.001) | n.s. | <.0001 | <.0001 | n.s. |
| CSF t-tau mean (SD) | 302 (99) | 293 (97) | 487 (249) | 543 (284) | χ2 = 15.9, η2 = .18 (<.001) | n.s. | <.05 | <.05 | n.s. |
| CSF p-tau mean (SD) | 50 (12) | 52 (14) | 74 (33) | 82 (44) | χ2 = 12.6, η2 = .14 (<.0001) | n.s. | <.05 | <.05 | n.s. |
| A + T−N− n (%) | 9 (50%) | 11 (52%) | † | † | † | † | † | ||
| A + T−N+ n (%) | 2 (11%) | 2 (10%) | † | † | † | † | † | ||
| A + T+N+ n (%) | 7 (39%) | 8 (38%) | † | † | † | † | † | ||
| APOE-ε4 n (%) | 0 (0 %) | 16 (100%) | 13 (72%) | 15 (74%) | † | † | † | † | † |
Abbreviations: n.s., nonsignificant result; Aβ+, CSF confirmed amyloid pathology; APOE-ε4+/−, apolipoprotein E 4 allele positive or negative; SCD, subjective cognitive decline; MCI, mild cognitive impairment; SD, standard deviation; ANOVA, analysis of variance; MMSE, Mini Mental State Examination; TMT, trail-making test; AD, Alzheimer's disease; CSF, cerebrospinal fluid.
No contrasts/post hoc tests performed.
No statistical tests applied.
2.5. Neuropsychological battery
The neuropsychological battery included the Mini Mental State Examination (MMSE-NR) [32], verbal learning and memory recall (CERAD word list test) [33], psychomotor speed, and divided attention (trail-making test A and B [TMT A and B]). T-scores for the trail-making tests were calculated using published norms [34]. For the CERAD word list test, we used the normative performance of the DDI cohort control group [26] to calculate T-scores after a recent article that showed published norms not matching the younger and more educated DDI cohort [35]. A total of 42 of 74 baseline cases had available cognitive data at 2-year follow-up.
2.6. Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed at 7 sites, and 7 scanners were used; a total of 57 MRI scans were available for analysis. For group 1 (12 subjects), MRI was performed on a Philips Achieva 3 Tesla system (Philips Medical Systems, Best, the Netherlands). A 3D T1-weighted turbo field echo sequence (TR/TE/TI/FA = 4.5 ms/2.2 ms/853 ms/8° matrix = 256 × 213, 170 slices, thickness = 1.2 mm, in-plane resolution of 1 mm × 1.2 mm) was obtained. For group 2 (22 subjects), MRI was performed using a Philips Ingenia 3 Tesla system (Philips Medical Systems, Best, the Netherlands). A 3D T1-weighted turbo field echo sequence (TR/TE/TI/FA = 4.5 ms/2.2 ms/853 ms/8°, matrix = 256 × 213, 170 slices, thickness = 1.2 mm, in-plane resolution of 1 mm × 1.2 mm) was obtained. For group 3 (3 subjects), MRI was performed using a Siemens Skyra 3 Tesla system (Siemens Medical Solutions, Erlangen, Germany). A 3D T1 magnetization-prepared rapid gradient–echo sequence (TR/TE/TI/FA = 2300 ms/2.98 ms/900 ms/9° matrix = 256 × 256, 176 slices, thickness = 1.2 mm, in-plane resolution of 1.0 mm × 1.0 mm) was obtained. For group 4 (11 subjects), MRI was performed using a Philips Ingenia 1.5 Tesla system (Philips Medical Systems, Best, the Netherlands). A 3D T1-weighted turbo field echo sequence (TR/TE/TI/FA = 7.63 ms/3.49 ms/937 ms/8° matrix = 256 × 256, 180 slices, thickness = 1.0 mm, in-plane resolution of 1.0 mm × 1.0 mm) was obtained. For group 5 (1 subject), MRI was performed using a Siemens Avanto 1.5 Tesla system (Siemens Medical Solutions, Erlangen, Germany). A 3D T1-weighted magnetization-prepared rapid gradient–echo sequence (TR/TE/TI/FA = 1190 ms/3.10 ms/750 ms/15° matrix = 512 × 512, 144 slices, thickness = 1.0 mm, in-plane resolution of 0.50 mm × 0.50 mm) was obtained. For group 6 (7 subjects), MRI was performed using a GE Optima Medical Systems 1.5 Tesla system (GE Healthcare, Chicago, IL). A 3D T1-weighted fast spoiled gradient–echo sequence (TR/TE/TI/FA = 11.26 ms/5.04 ms/500 ms/10° matrix = 256 × 256, 156 slices, thickness = 1.2 mm, in-plane resolution of 1.0 mm × 1.0 mm) was obtained. Finally, 1 MRI scan was performed using a Siemens Avanto 1.5 Tesla system (Siemens Medical Solutions, Erlangen, Germany). A 3D T1-weighted magnetization-prepared rapid gradient–echo sequence (TR/TE/TI/FA = 1700 ms/2.42 ms/1000 ms/15° matrix = 256 × 256, 144 slices, thickness = 1.2 mm, in-plane resolution of 1.0 mm × 1.0 mm) was obtained.
2.7. MRI segmentations and analyses
Volumetric segmentation was performed with the FreeSurfer image analysis suite version 6.0.0 (http://surfer.nmr.mgh.harvard.edu/). This includes segmentation of the subcortical white matter and deep gray matter volumetric structures [36]. For the hippocampus and amygdala, volumes from the left and right hemispheres were added, and relative volumes (per mL of total intracranial volume) were computed.
2.8. Statistical analysis
Normality was assessed through the inspection of QQ-plots, histograms, and the Shapiro-Wilk test of normality.
To assess differences in biomarker levels, MRI-derived medial temporal lobe (MTL) volumes, cognitive tests, and demographics between groups, we performed one-way analyses of variance (ANOVAs) with planned comparisons for variables with normal distributions. For MTL volumes, ANOVA analyses were performed on standardized residuals after covariate regression correction for age, gender, and MRI scanner model. We performed Kruskal-Wallis test with Dunn's nonparametric pairwise post hoc test to assess group differences in variables with non-normal distributions (CSF Aβ1–42, CSF t-tau, CSF t-tau, CERAD recall T-score, and MMSE). Nonparametric pairwise comparisons and ANOVA contrasts were performed in a hierarchical manner. If the high- and low-risk control groups were found equal on the relevant measure, we proceeded to compare SCD and MCI groups to controls (collapsed control group) and finally comparing the SCD with the MCI group. The dichotomous variable “gender” was assessed using a chi-square test. To compare levels of CSF neurogranin, CSF BACE1, and their ratio score to groups derived from the A/T/N groups, one-way ANOVAs with post hoc Bonferroni corrections were performed. Effect sizes are provided for ANOVA (ηp2) and Kruskal-Wallis test (η2) [37].
The impact of CSF biomarkers on MMSE scores were assessed using a multiple linear regression model controlling for age, and simple linear regression models were fitted to assess the relationship between biomarkers and age-adjusted T-scores for the different cognitive tests at baseline. Similarly, the relationships between biomarkers and MTL volumes were assessed using several multiple regression analyses controlling for effects of age, gender, and MRI scanner variant. Effect sizes for the overall regression models are provided (R2).
Because CSF Aβ1–42 was used as core selection criteria in the study design, it was omitted as predictor from baseline regression analyses with cognitive and MRI variables. However, we assessed CSF Aβ1–42 as the predictor of cognitive changes at 2-year follow-up. CSF p-tau and t-tau demonstrated collinearity (variance inflation factor > 7). Thus, only CSF total tau was included in our regression models.
To assess the individual change in cognitive scores between baseline and 2-year follow-up, individual follow-up scores were subtracted from baseline scores. The resulting score was used to predict cognitive changes from baseline CSF biomarkers using linear regression models.
All analyses were performed in the Statistical Package for Social Sciences (SPSS) version 24.
2.9. Ethics
The regional medical research ethics committee approved the study. Participants gave their written informed consent before taking part in the study. All further study conduct was in line with the guidelines provided by the Helsinki declaration of 1964, revised 2013 and the Norwegian Health and Research act.
3. Results
3.1. Between-group CSF biomarker comparisons
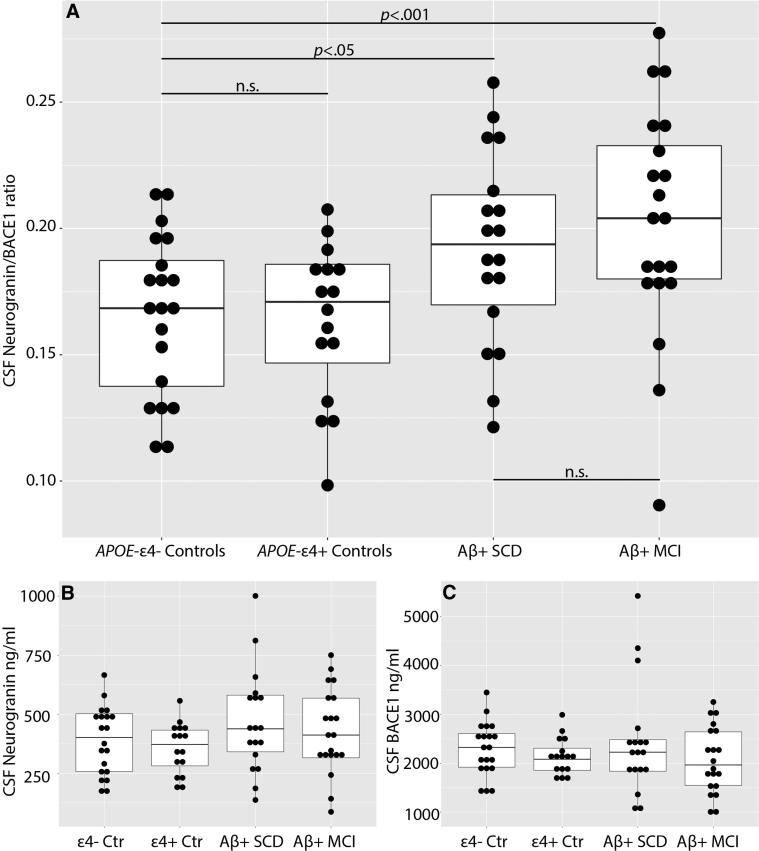
We found significantly increased levels of CSF Ng/BACE1 in both SCD (t(71) = 2.532, P < .05) and MCI (t(71) = 3.595, P < .001) compared with controls. No differences were demonstrated between SCD and MCI groups or even between the high- vs. low-risk control groups (Table 2 and Fig. 1). Moreover, no significant between-group differences were found for Ng or for BACE1 when measured separately (Table 2).
Table 2.
Between-group comparisons between CSF biomarkers and MTL volumetry
| Variable | Groups |
F and ηp2(P) | ANOVA contrasts (P) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| APOE-ε4− controls (n = 20) | APOE-ε4+ controls (n = 16) | Aβ+ SCD (n = 18) | Aβ+ MCI (n = 20) | 1 vs 2 | 3 vs 1 and 2 | 4 vs 1 and 2 | 3 vs 4 | ||
| CSF Ng mean (SD) | 390 (143) | 355 (108) | 468 (217) | 428 (179) | F = 1.5 (n.s) | ∗ | ∗ | ∗ | ∗ |
| CSF BACE1 mean (SD) | 2289 (547) | 2140 (374) | 2442 (1132) | 2064 (679) | F = 0.4 (n.s) | ∗ | ∗ | ∗ | ∗ |
| CSF Ng/BACE1 mean (SD) | .1659 (.03) | .1635 (.03) | .1921 (.04) | .2022 (.05) | F = 4.9, ηp2 = .17 (<.01) | n.s. | <.05 | <.01 | n.s. |
| Hippocampus average volume mean (SD) | 23.0 (2.9) | 22.4 (3.9) | 21.4 (3.3) | 19.4 (3.8) | F = 2.3† (n.s.) | ∗ | ∗ | ∗ | ∗ |
| Amygdala average volume mean (SD) | 1.2 (0.3) | 1.1 (0.2) | 1.0 (0.2) | 0.9 (0.2) | F = 1.8† (n.s.) | ∗ | ∗ | ∗ | ∗ |
Abbreviations: n.s., nonsignificant result; Aβ+, CSF confirmed amyloid pathology; APOE-ε4+/−, apolipoprotein E 4 allele positive or negative; CSF, cerebrospinal fluid; MTL, medial temporal lobe; ANOVA, analysis of variance; Ng, neurogranin; SD, standard deviation; BACE1, β-site amyloid precursor protein-cleaving enzyme 1.
Contrasts or post hoc tests not performed due to non-significant ANOVA.
Between-group comparisons of MRI medial temporal volumetry are performed on standardized residuals following covariate regression correction for age, gender and MRI scanner variant.
Fig. 1.
Ng/BACE1 ratio (A), CSF Ng level (B), and CSF BACE1 level (C) between groups. Abbreviations: CSF, cerebrospinal fluid; Ng, neurogranin; BACE1, β-site amyloid precursor protein-cleaving enzyme 1; Ctr, controls; APOE-ε4+/−, apolipoprotein E4 allele positive or negative; Aβ+, CSF amyloid pathology; SCD, subjective cognitive decline; MCI, mild cognitive impairment. Horizontal brackets showing contrast comparisons for CSF Ng/BACE1 only (A). Significant results (P < .05) or nonsignificant results (n.s.) are shown.
3.2. CSF biomarkers in relation to A/T/N groups
Both CSF Ng (F(3,69) = 8.801, ηp2 = .28, P < .0001) and CSF BACE1 (F(3,69) = 7.201, ηp2 = .24, P < .0001), as well as CSF Ng/BACE1 ratio (F(3,69) = 6.656, ηp2 = .22, P < .0001), were significantly different between A/T/N groups.
Levels of CSF Ng/BACE1 were increased in the A+ N+ group (n = 10, M = .2102, standard deviation [SD] = .05) compared with controls (n = 35, M = .1642, SD = .03, P < .01). However, this was not shown for Ng or BACE1 when measured separately. Both CSF BACE1 (n = 13, M = 2884, SD = 958, P < .05) and Ng levels (M = 580, SD = 164, P < .0001), as well as Ng/BACE1 level (M = .2061, SD = .04, P < .01), were elevated in the A + T+N+ group compared with individuals with normal CSF (Ng: M = 369, SD = 126; Ng/BACE1: M = .1642, SD = .03). In addition, Ng (n = 13, M = 580, SD = 164) was also elevated in the A + T+N+ group compared with the A+ group (n = 15, M = 323, SD = 129, P < .0001). No significant differences between healthy controls with normal CSF and amyloid-positive (A+) individuals were found for CSF BACE1, Ng, or Ng/BACE1.
3.3. CSF biomarkers, APOE-ε4, and MRI-derived medial temporal volumetry
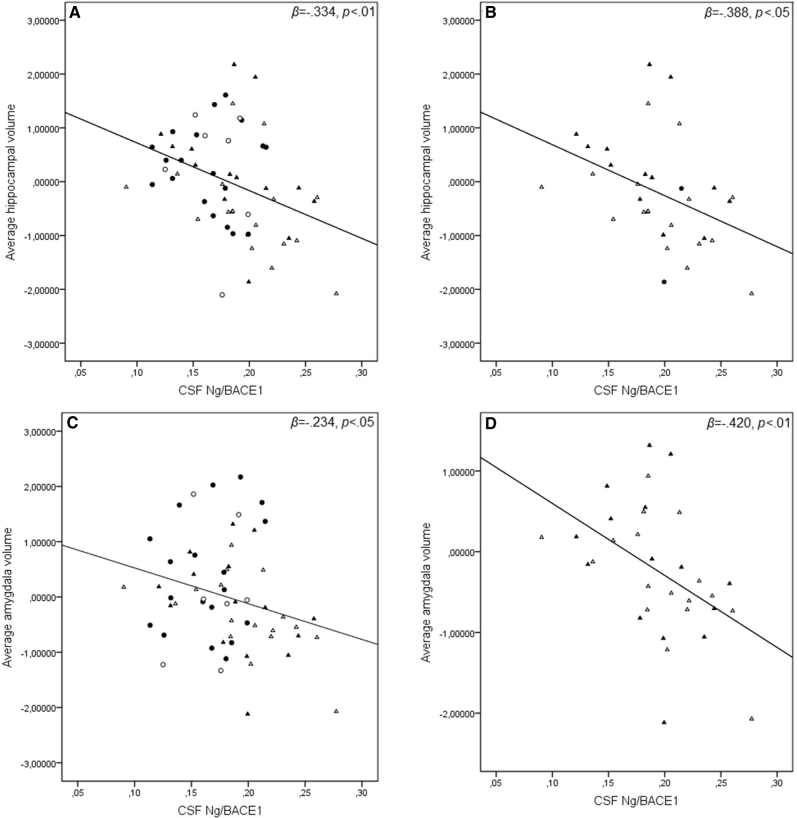
All models include covariates controlling for age, gender, and scanner variant. When analyzing the entire sample (n = 57), higher CSF Ng/BACE1 levels were associated with reduced average hippocampal volume (β = −.334, P < .01, adjusted R2 = 0.410, F(4,53) = 9.225, P < .0001). Similarly, higher CSF Ng/BACE1 was associated with reduced average amygdala volume (β = −.234, P < .05, adjusted R2 = 0.369, F(4,53) = 9.230, P < .0001). When the amyloid-positive subjects (SCD and MCI, n = 31) were analyzed separately, higher CSF Ng/BACE1 was significantly associated with reductions in both hippocampal (β = −.388, P < .05, adjusted R2 = 0.350, F(4,27) = 5.175, P < .01) and amygdala volumes (β = −.420, P < .01, adjusted R2 = 0.502, F(4,27) = 8.814, P < .0001) (Effects are depicted in Fig. 2). No other associations between CSF biomarkers or APOE-ε4 carrier status and MTL volumetry were found. Significant regression coefficients are shown in Table 3. No overall significant differences in average hippocampal or amygdala volumes between groups were found. Please see Table 2 for details.
Fig. 2.
CSF Ng/BACE1 in relation to medial temporal lobe volumetry. Average hippocampal (A & B) and amygdala volumes (C & D). Medial temporal lobe volumes are adjusted for age, gender, and MRI scanner variant. Open circles = APOE-ε4+ controls. Closed circles = APOE-ε4− controls. Open triangles = MCI with amyloid plaques. Closed triangles = SCD with amyloid plaques. Abbreviations: CSF, cerebrospinal fluid; Ng, neurogranin; BACE1, β-site amyloid precursor protein-cleaving enzyme 1; APOE-ε4+/−, apolipoprotein E4 allele positive or negative; SCD, subjective cognitive decline; MCI, mild cognitive impairment.
Table 3.
Regression coefficients between biomarkers, MTL volumes, and cognitive tests at baseline and difference in T-score at 2-year follow-up
| Variable | CSF Ng |
CSF BACE1 |
CSF Ng/BACE1 |
CSF t-tau |
CSF Aβ1–42 |
APOE-ε4 allele positivity |
|---|---|---|---|---|---|---|
| Biomarker and MTL measures entire sample (n = 57)/Aβ+ SCD and Aβ+ MCI (n = 30) | ||||||
| Amygdala | ∗/∗ | ∗/∗ | β = −.234† / β = −420†; P < .05 / P < .01 | ∗/∗ | § | ∗/∗ |
| Hippocampus |
∗/∗ |
∗/∗ |
β = −.334† / β = −.388†; P < .01 / P < .05 |
∗/∗ |
§ |
∗/∗ |
| Biomarkers and Baseline cognitive tests (N = 74)/Biomarkers and cognitive change at 2-year follow-up (n = 42) |
||||||
| MMSE | ∗/∗ | ∗/∗ | β = −.258‡ / β = −.312; P < .05 / P < .05 | ∗/∗ | § | ∗/∗ |
| CERAD learning T-score | ∗/β = −.322; ∗/ P < .05 | ∗/∗ | β = −.266 / β = −.352; P < .05 / P < .05 | ∗/β = −.413; ∗/ P < .01 | § | ∗/∗ |
| CERAD recall T-score | ∗/∗ | ∗/∗ | β = −.312/∗P < .05/∗ | ∗/∗ | § | ∗/∗ |
| TMT-A T-score | ∗/∗ | ∗/∗ | β = −.238/∗P < .05/∗ | ∗/∗ | § | ∗/∗ |
| TMT-B T-score | ∗/∗ | ∗/∗ | ∗/∗ | ∗/∗ | § | ∗/∗ |
Abbreviations: CSF, cerebrospinal fluid; Ng, neurogranin; MTL, medial temporal lobe; BACE1, β-site amyloid precursor protein-cleaving enzyme 1; APOE-ε4+/−, apolipoprotein E 4 allele positive or negative; SCD, subjective cognitive decline; MCI, mild cognitive impairment; CERAD, the Consortium to Establish a Registry for Alzheimer's Disease word list test; MMSE, Mini Mental State Examination; TMT, trail-making test; MRI, magnetic resonance imaging.
Nonsignificant result.
Model includes age, gender, and MRI scanner variant as covariate.
Model includes age as covariate.
Not performed at baseline due to study design selection bias.
3.4. CSF biomarkers and APOE-ε4 in relation to baseline cognitive performance
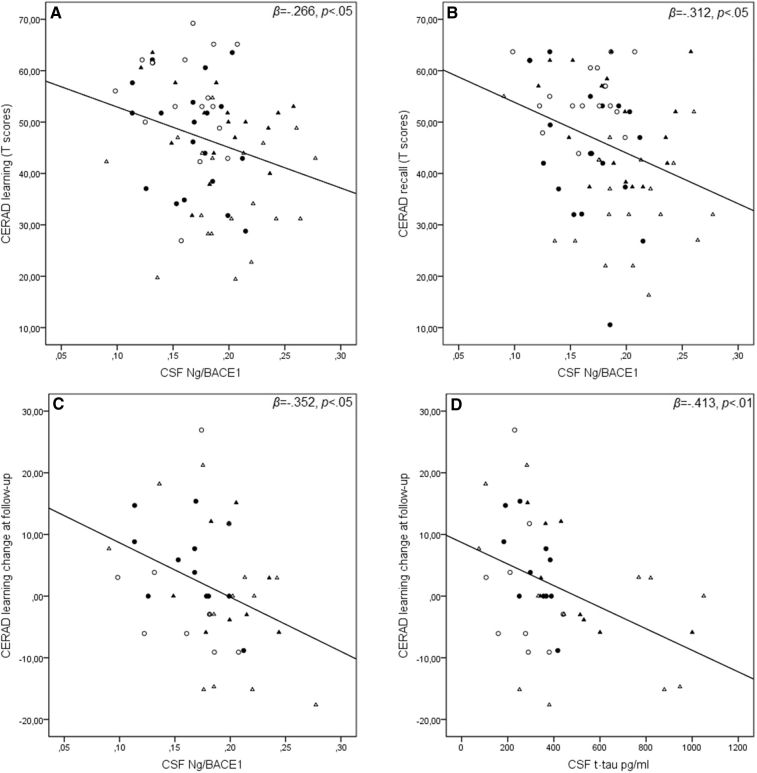
We found a significant inverse relationship between higher CSF Ng/BACE1 and lower performance in CERAD learning T-score (R2 = 0.71, F(1,70) = 5.321, β = −.266, P < .05); CERAD recall T-score (R2 = 0.97, F(1,70) = 7.535, β = −.312, P < .01); and TMT-A T-score (R2 = .057, F(1,70) = 4.153, β = −.238, P < .05) (effect shown in Fig. 3). Moreover, when controlling for age (β = −.124, P = .31), we found that higher Ng/BACE1 (β = −.258, P < .05) also was associated with lower scores on the MMSE (adjusted R2 = 0.78, F(2,70) = 4.044, P < .05).
Fig. 3.
CSF Ng/BACE1 and CSF t-tau in relation to baseline and 2-year follow-up CERAD learning and memory recall tests. CSF Ng/BACE1 and baseline CERAD subtest T-scores (A & B). CERAD Learning T-score change at follow-up CSF Ng/BACE1 (C) and CSF t-tau (D). Open circles = APOE-ε4+ controls. Closed circles = APOE-ε4− controls. Open triangles = MCI with amyloid plaques. Closed triangles = SCD with amyloid plaques. Abbreviations: CERAD, the Consortium to Establish a Registry for Alzheimer's Disease word list test; CSF, cerebrospinal fluid; Ng, neurogranin; BACE1, β-site amyloid precursor protein-cleaving enzyme 1; APOE-ε4+/−, apolipoprotein E4 allele positive or negative; SCD, subjective cognitive decline; MCI, mild cognitive impairment.
No relationships between baseline cognitive measures and APOE-ε4 carrier status or other CSF biomarkers were demonstrated. Statistically significant relationships were only found when analyzing the entire sample and are summarized in Table 3.
3.5. Baseline CSF biomarkers and APOE-ε4 carrier status predicting change in cognitive performance at 2-year follow-up
Lower baseline CSF Ng/BACE1 levels predicted practice effects (i.e., showing improved performance between baseline and follow-up), whereas increasing levels predicting less improvement and finally a decline between assessments in both CERAD learning T-score (R2 = 0.124, F(1,40) = 5.646, β = −.352, P < .05) and MMSE (R2 = 0.97, F(1,42) = 4.426, β = −.312, P < .05). A similar result was also obtained for Ng measured separately but only relating to the CERAD learning T-score (R2 = 0.104, F(1,40) = 4.622, β = −.322, P < .05). Similarly, CSF t-tau significantly predicted cognitive decline in CERAD learning (R2 = 0.170, F(1,40) = 8.217, β = −.413, P < .01) (effects are illustrated in Fig. 3). No relationships between 2-year cognitive change, APOE-ε4 carrier status, or other baseline CSF biomarkers were found. Significant relationships between baseline biomarkers and follow-up cognitive performance are summarized in Table 3.
4. Discussion
To our knowledge, this is the first study showing that Ng/BACE1 level is increased already at a preclinical stage of AD. Ng/BACE1 levels were equally increased in both Aβ+ MCI and SCD groups compared with controls, and no difference in Ng/BACE1 levels between APOE-ɛ4 +/− controls were found. Increased Ng/BACE1 level was the only marker related to baseline hippocampal and amygdala volumes in our sample. Concordantly, the Ng/BACE1 level was the only biomarker associated with poorer baseline performance in both baseline CERAD learning and memory recall, as well as attention/psychomotor speed (TMT-A) and global cognitive function (MMSE).
Furthermore, when analyzing available 2-year follow-up cognitive scores, we found that lower baseline Ng/BACE1 levels predicted practice effects in the CERAD learning subtest at follow-up (i.e., showing improved performance) and increasing ratios predicted less improvement and finally a decline in CERAD word list–learning ability. This relationship was also shown for CSF Ng measured separately, supporting previous findings [2], [4]. Although a similar result was obtained with CSF t-tau as the baseline predictor, an inspection of the scatter plot indicated that the regression model may have been biased by a few subjects with extreme baseline CSF total tau values. This result suggests that the subjects with high baseline measures of neuronal degradation (CSF t-tau) may be at a more advanced stage of disease development and therefore show a steeper cognitive decline. This is in line with findings linking markers of neuronal degradation to disease severity [38]. In contrast, Ng/BACE1 levels may represent synaptic loss that is more closely tied to smaller increments of cognitive decline along the early Alzheimer's trajectory, which may precede markers of significant neuronal degradation. This could explain why only the Ng/BACE1 level was related to baseline learning and memory function in our sample, possibly due to early synaptic loss in the hippocampus where neurogranin is highly expressed [7]. Moreover, although a higher Ng/BACE1 level was related to lower MMSE at baseline and decline at follow-up both in our previous [21] and present studies, Ng/BACE1 level was predominantly related to CERAD learning and memory recall. The MMSE contains word list memory items, and the observed relationship could be influenced by this shared measure. Interestingly, TMT-A, a measure of psychomotor speed and attention, was inversely related to CSF Ng/BACE1 level. This is in accordance with previous investigations showing that performance on the TMT-A is related to amyloid load in SCD cases and mixed samples of MCI and healthy subjects [39], [40].
BACE1 and neurogranin have predominantly presynaptic [9], [10] and postsynaptic roles, and neurogranin, in particular, is linked to the dendritic spine NMDA Ca2+-Calmodulin second messenger complex [8]. Although synapse degeneration per se is not disease specific, the link between Aβ oligomerization, NMDA disruption, and spine Ca2+-dysregulation [11], [13] may confer an AD specificity to the Ng/BACE1 ratio marker and point to a postsynaptic Aβ-linked disease mechanism. This further strengthens the suggestion that NMDA antagonists may be protective in AD [41]. In this scenario, enhanced synaptotoxic polymerization of Aβ-peptides in APOE-ɛ4 SCD and MCI cases will have a more rapid synaptic loss due to increased levels of synaptotoxic Aβ fibrils [11], [14], [15]. Although APOE-ɛ4 carrier status did not significantly relate to medial temporal volumes or cognition in our sample, a large majority of the Aβ+ SCD and MCI cases (28 of 37) had at least one APOE-ɛ4 allele. Moreover, APOE-ɛ4 carriers with amyloid plaques had higher CSF Ng/BACE1 levels than noncarriers with plaques (data not shown). The Ng/BACE ratio was shown to increase with A/T/N-classified AD biomarker severity (i.e., moving from normal CSF toward amyloid plaques combined with markers of neurodegeneration and neurofibrillary tangles) [19]. An increase was also observed for both CSF BACE1 [20] and Ng [21] separately, supporting previous findings indicating a link to neurodegeneration. Though APOE-ɛ4 could enhance Ng/BACE1-related pathology through its interaction with Aβ [11], [14], [15], a larger material with more APOE-ɛ4− and Aβ+ SCD and MCI cases will be needed to establish ɛ4-allelic effects.
Both the link to cognitive measures and strong associations to volume reductions in pertinent MTL structures lend further support to a putative role of Ng/BACE1 as a biomarker for Alzheimer-related synaptic loss. CSF Ng/BACE1 level was similarly increased in the Aβ+ MCI and SCD groups, thus the SCD cases may harbor an active disease state, including progressive synaptic loss, experienced as a SCD that has yet to reach the threshold for clinical impairment.
Some limitations of this study need to be addressed. First, care must be taken in interpreting these findings due to a relatively small baseline sample size (n = 74), confined to small subgroups, and the even smaller sample size with available cognitive tests at a relatively short 2-year follow-up interval (n = 42). This may explain why we did not show an expected association between CSF Ng and hippocampal volume in our sample [2], [4] or expected between-group differences in MTL atrophy in amyloid-positive subjects [42], [43]. Second, although the National Institute on Aging and Alzheimer's Association (NIA-AA) [28] recommends an MCI cutoff value of between −1 and −1.5 SD below the mean, we opted for a stringent cutoff at ≤−1.5 SD which can impact SCD/MCI group classification. However, cognitive performance in the SCD group was similar to that in the control group in our study, indicating that the SCD group's cognitive performance was within the normal range. Finally, we did not include Aβ-negative SCD or MCI cases or explore potential differences between homozygote and heterozygote APOE-ɛ4 carriers to other APOE genotypes; both of which we plan to explore in subsequent articles.
4.1. Conclusions
To our knowledge, this is the first study showing that the Ng/BACE1 ratio is related to memory deficits and reduced MTL volumes in Aβ-positive preclinical cases and that Ng/BACE1 is significantly increased relative to controls in amyloid-positive subjects with SCD. These results warrant further studies investigating the role of Ng/BACE1 in the AD pathogenesis, potentially reflecting synaptic pathology due to an Aβ-linked disease mechanism. Although NMDA antagonists have been suggested to be protective [36], the present findings suggest that such intervention guided by an early Ng/BACE1 increase might be useful.
Research in context.
-
1.
Systematic review: Synapse loss occurs early in Alzheimer's disease (AD). Increased CSF neurogranin (Ng) is related to synapse loss and β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is involved in presynaptic amyloid-β precursor protein metabolism. Previously, we found that an increased Ng/BACE1 ratio predicted cognitive decline in predementia AD. This ties in with the findings linking reduced beta-amyloid clearance to postsynaptic spine affection in early AD. Here, we investigate CSF Ng/BACE1 level as a preclinical marker of synapse loss in AD.
-
2.
Interpretation: We found higher CSF Ng/BACE1 levels in preclinical and predementia AD related to reduced hippocampal volume and memory function at baseline and cognitive decline at follow-up. These results lend support to Ng/BACE1 as an early marker of synaptic loss in AD, which is sensitive also for preclinical changes.
-
3.
Future directions: A high Ng/BACE1 ratio may point to the AD-related damage of postsynaptic spines. If confirmed, this could indicate specific early intervention measures and show target engagement in intervention studies.
Acknowledgments
The project was funded by Norwegian Research Council, NASATS (Dementia Disease Initiation), and the JPND (APGeM) and funding from the regional health authorities (Helse Sør-Øst and Helse Nord). This article represents independent research (part) funded by the National Institute for Health Research (NIHR) Biomedical Research Center at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. The authors thank Marianne Wettergren (Akershus University hospital, Norway) for essential help with CSF analysis and Erna Utnes (Akershus University hospital, Norway) for assistance with data collection and handling.
Footnotes
Declarations of Interest: K.N. receives publishing royalties from the popular science books “Hjernen er stjernen” from 2016 and “Hjernetrening” from 2018 published at Kagge Forlag AS (Oslo, Norway) and gets honoraria for talks with the same title from Athenas speakers' bureau (Tønsberg, Norway). B.B. is a full-time employee of EUROIMMUN AG. E.V. is the co-founder of ADx NeuroSciences, Zwijnaarde, Belgium, and founder of Key4AD, Eke, Belgium. D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, and GE Health, and serves as paid consultant for H. Lundbeck, Eisai, and Axovant. D.A. is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation and the Royal Society for their support. Other authors have no conflicts of interest to disclose.
References
- 1.Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004;23:105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- 2.Tarawneh R., D'Angelo G., Crimmins D., Herries E., Griest T., Fagan A.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016;73:561–571. doi: 10.1001/jamaneurol.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellington H., Paterson R.W., Portelius E., Tornqvist U., Magdalinou N., Fox N.C. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86:829–835. doi: 10.1212/WNL.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portelius E., Zetterberg H., Skillback T., Tornqvist U., Andreasson U., Trojanowski J.Q. Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015;138:3373–3385. doi: 10.1093/brain/awv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kester M.I., Teunissen C.E., Crimmins D.L., Herries E.M., Ladenson J.H., Scheltens P. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA Neurol. 2015;72:1275–1280. doi: 10.1001/jamaneurol.2015.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vos A., Jacobs D., Struyfs H., Fransen E., Andersson K., Portelius E. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimers Dement. 2015;11:1461–1469. doi: 10.1016/j.jalz.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Higo N., Oishi T., Yamashita A., Matsuda K., Hayashi M. Cell type- and region-specific expression of neurogranin mRNA in the cerebral cortex of the macaque monkey. Cereb Cortex. 2004;14:1134–1143. doi: 10.1093/cercor/bhh073. [DOI] [PubMed] [Google Scholar]
- 8.Diez-Guerra F.J. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life. 2010;62:597–606. doi: 10.1002/iub.357. [DOI] [PubMed] [Google Scholar]
- 9.Del Prete D., Lombino F., Liu X., D'Adamio L. APP is cleaved by Bace1 in pre-synaptic vesicles and establishes a pre-synaptic interactome, via its intracellular domain, with molecular complexes that regulate pre-synaptic vesicles functions. PLoS One. 2014;9:e108576. doi: 10.1371/journal.pone.0108576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J., Roy S. The physical approximation of APP and BACE-1: A key event in alzheimer's disease pathogenesis. Dev Neurobiol. 2018;78:340–347. doi: 10.1002/dneu.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberdi E., Sanchez-Gomez M.V., Cavaliere F., Perez-Samartin A., Zugaza J.L., Trullas R. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Li P., Feng J., Wu M. Dysfunction of NMDA receptors in Alzheimer's disease. Neurol Sci. 2016;37:1039–1047. doi: 10.1007/s10072-016-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzheimer's Association Calcium Hypothesis W Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 2017;13:178–182.e17. doi: 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Sanan D.A., Weisgraber K.H., Russell S.J., Mahley R.W., Huang D., Saunders A. Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh T.V., Davis A.A., Ulrich J.D., Holtzman D.M. Apolipoprotein E and Alzheimer's disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. J Lipid Res. 2017;58:824–836. doi: 10.1194/jlr.R075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer's disease. Alzheimer's Res Ther. 2014;6:89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barao S., Moechars D., Lichtenthaler S.F., De Strooper B. BACE1 Physiological Functions May Limit Its Use as Therapeutic Target for Alzheimer's Disease. Trends Neurosci. 2016;39:158–169. doi: 10.1016/j.tins.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Egan M.F., Kost J., Tariot P.N., Aisen P.S., Cummings J.L., Vellas B. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer's Disease. N Engl J Med. 2018;378:1691–1703. doi: 10.1056/NEJMoa1706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barao S., Zhou L., Adamczuk K., Vanhoutvin T., van Leuven F., Demedts D. BACE1 levels correlate with phospho-tau levels in human cerebrospinal fluid. Curr Alzheimer Res. 2013;10:671–678. doi: 10.2174/15672050113109990138. [DOI] [PubMed] [Google Scholar]
- 21.De Vos A., Struyfs H., Jacobs D., Fransen E., Klewansky T., De Roeck E. The Cerebrospinal Fluid Neurogranin/BACE1 Ratio is a Potential Correlate of Cognitive Decline in Alzheimer's Disease. J Alzheimers Dis. 2016;53:1523–1538. doi: 10.3233/JAD-160227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janelidze S., Pannee J., Mikulskis A., Chiao P., Zetterberg H., Blennow K. Concordance Between Different Amyloid Immunoassays and Visual Amyloid Positron Emission Tomographic Assessment. JAMA Neurol. 2017;74:1492–1501. doi: 10.1001/jamaneurol.2017.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 24.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 25.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fladby T., Palhaugen L., Selnes P., Waterloo K., Brathen G., Hessen E. Detecting At-Risk Alzheimer's Disease Cases. J Alzheimers Dis. 2017;60:97–105. doi: 10.3233/JAD-170231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reijs B.L., Teunissen C.E., Goncharenko N., Betsou F., Blennow K., Baldeiras I. The Central Biobank and Virtual Biobank of BIOMARKAPD: A Resource for Studies on Neurodegenerative Diseases. Front Neurol. 2015;6:216. doi: 10.3389/fneur.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjogren M., Vanderstichele H., Agren H., Zachrisson O., Edsbagge M., Wikkelso C. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 31.Kalheim L.F., Fladby T., Coello C., Bjrnerud A., Selnes P. [18F]-Flutemetamol Uptake in Cortex and White Matter: Comparison with Cerebrospinal Fluid Biomarkers and [18F]-Fludeoxyglucose. J Alzheimers Dis. 2018;62:1595–1607. doi: 10.3233/JAD-170582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Fillenbaum G.G., van Belle G., Morris J.C., Mohs R.C., Mirra S.S., Davis P.C. Consortium to Establish a Registry for Alzheimer's Disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4:96–109. doi: 10.1016/j.jalz.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaton R.K. Psychological Assessment Resources; Lutz, Florida: 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults : Professional Manual. [Google Scholar]
- 35.Kirsebom B.E., Espenes R., Waterloo K., Hessen E., Johnsen S.H., Brathen G. Screening for Alzheimer's Disease: Cognitive Impairment in Self-Referred and Memory Clinic-Referred Patients. J Alzheimers Dis. 2017;60:1621–1631. doi: 10.3233/JAD-170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 37.Fritz C.O., Morris P.E., Richler J.J. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 38.Sämgård K., Zetterberg H., Blennow K., Hansson O., Minthon L., Londos E. Cerebrospinal fluid total tau as a marker of Alzheimer's disease intensity. Int J Geriatr Psychiatry. 2010;25:403–410. doi: 10.1002/gps.2353. [DOI] [PubMed] [Google Scholar]
- 39.Duara R., Loewenstein D.A., Shen Q., Barker W., Potter E., Varon D. Amyloid positron emission tomography with (18)F-flutemetamol and structural magnetic resonance imaging in the classification of mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2013;9:295–301. doi: 10.1016/j.jalz.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loewenstein D.A., Curiel R.E., Greig M.T., Bauer R.M., Rosado M., Bowers D. A Novel Cognitive Stress Test for the Detection of Preclinical Alzheimer Disease: Discriminative Properties and Relation to Amyloid Load. Am J Geriatr Psychiatry. 2016;24:804–813. doi: 10.1016/j.jagp.2016.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R., Reddy P.H. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J Alzheimers Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pini L., Pievani M., Bocchetta M. Brain atrophy in Alzheimer's Disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Zhou M., Zhang F., Zhao L., Qian J., Dong C. Entorhinal cortex: a good biomarker of mild cognitive impairment and mild Alzheimer's disease. Rev Neurosci. 2016;27:185–195. doi: 10.1515/revneuro-2015-0019. [DOI] [PubMed] [Google Scholar]