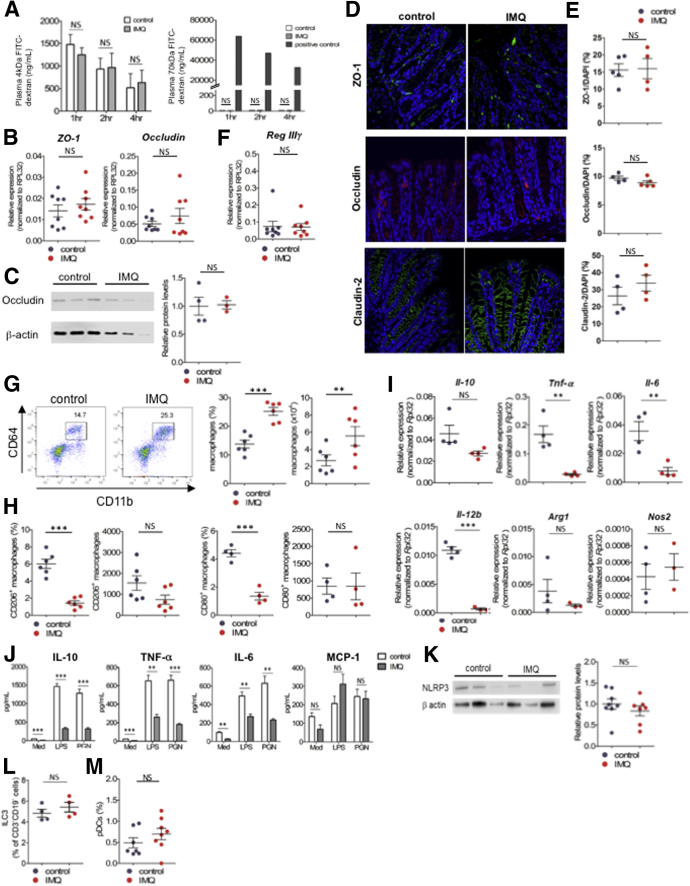
Figure 2.
IMQ dermatitis mice did not increase intestinal permeability but altered immune cell composition in the intestine. (A) Analysis for intestinal permeability of mice induced psoriasis-like dermatitis (on day 17 of IMQ or vehicle treatment), by using 4 kilodaltons (left) or 70 kilodaltons (right) FITC-dextran. Plasma FITC-dextran concentrations were determined by fluorescence intensity, after 1, 2 and 4 hours from oral administration of FITC-dextran (n = 4, in each group). Seventy kilodaltons FITC-dextran was administered intravenously as a positive control. (B) Quantitative PCR analysis for ZO-1 and occludin in colonic epithelial cells after topical IMQ treatment (on day 17) analyzed by real-time PCR. (C) Western blot analysis for occludin expression in colonic epithelial cells after topical IMQ treatment (on day 17). Western blot (left) and relative protein levels (right) of occludin. (D) Immunofluorescence staining was performed in control and IMQ mice. ZO-1 (top, green), occludin (middle, red), claudin (bottom, green) stained with 4′,6-diamidino-2-phenylindole (DAPI; blue) in colon tissue. Four to 5 sections from each mice were obtained and the average positive area of each protein was compared with the DAPI-positive area measured (n = 4–5, each dot represents each mouse). (F) Quantitative PCR analysis for Reg IIIγ in colonic epithelial cells after topical IMQ treatment (on day 17) analyzed by real-time PCR. (G) Representative plots of flow cytometry analysis for macrophages (CD11b+CD64+ cells) in CD45+CD3-CD19- B220-NK1.1- colonic lamina propria after IMQ treatment (on day 17) (n = 6 in each group). Percentage (left) and absolute number (right) of macrophages in colonic lamina propria. (H and I) Percentage and absolute number of CD206+ (left) or CD80+ (right) macrophages. (I) Quantitative PCR analysis of IL10, Tnf-α, Il6, IL12b, Arg-1, and Nos2 in LP CD11b+s (n = 3–4 in each group). (J) CD11b+ cells were sorted by magnetic activated cell sorting from colonic lamina propria mononuclear cells on day 17 of IMQ treatment (LP CD11b+s). LP CD11b+s were cultured with lipopolysaccharide (LPS) or peptidoglycan (PGN) for 24 hours in vitro, and concentrations of cytokines in the supernatant were measured by cytometric bead array. Unstimulated control was cultured with medium (Med). (K) Western blot analysis for NLRP3 in peritoneal cavity cells, which are composed mainly of macrophages. Western blot (left) and relative protein levels (right) of NLRP3 (n = 7–8, pooled from 2 independent experiments, in each group). (L) Percentage of ILC3 in CD45+CD3-CD19- cells and (M) pDCs in CD45+CD11b-CD11c+ cells in colonic lamina propria (ILC3, CD45+CD3-CD19-Rorγt+ cells; pDC, CD45+CD11b-CD11c+B220+PDCA-1+ cells. Each symbol represents an individual mouse (n = 4–8). Statistical analyses were performed with the Student t test. *P < .05, **P < .01, ***P < .001. Error bars represent the SEM of samples within a group.