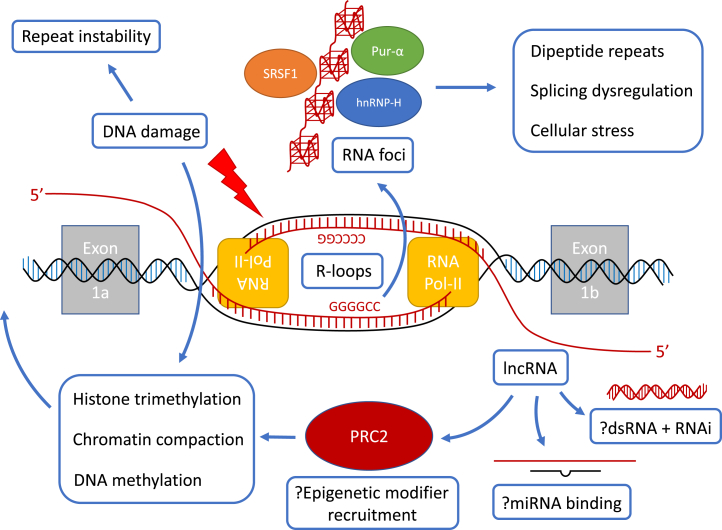
Fig. 3.
Potential non-coding RNA effects in C9orf72-related ALS/FTD. Bidirectional transcription across the (GGGGCC)n repeat expansion may lead to aberrant R-loop formation, predisposing the exposed DNA strands to damage, which in turn can lead to repeat instability through aberrant DNA repair. Bidirectional transcription may also lead to transcriptional interference between sense and antisense transcripts. Polymerase stalling may generate abortive transcripts and in the sense direction these can form G-quadruplex-containing RNA foci, which sequester RNA binding proteins such as SRSF1, Pur-α and hnRNP-H, leading to splicing dysregulation. Concurrently, repeat-containing transcripts undergo repeat-associated non-ATG dependent translation, forming dipeptide repeats that impact upon cellular stress at multiple levels such as through impaired nucleocytoplasmic transport and ubiquitin-proteasome function and through nucleolar stress. The natural antisense transcript of C9orf72 may induce downregulation through the RNAi pathway and may also potentially bind miRNAs relevant to neuronal function. Epigenetic modifiers such as the repressive PRC2 complex may be recruited to the locus, leading to histone trimethylation, chromatin compaction and DNA methylation.