Abstract
Currently available high-throughput technologies combined with bioinformatics analyses revealed that nearly 80% of the genome is transcribed, whereas only 2% of the genetic code is translated in proteins. In the landscape of non-coding RNA, the long non-coding RNA (>200 nucleotides) is a newer class of ncRNAs, with a potential pivotal role in homeostatic and pathological mechanisms, confirmed by increasing emerging evidences in different diseases, especially in cancer. In parallel, recent studies have demonstrated that as cancer progresses, extracellular matrix co-evolves into an activated state through continuous biochemical and structural modifications. In this review, we synthesize these themes by exploring the functional cross-talk between lncRNAs and their involvement in ECM regulation and remodeling within the tumor microenvironment.
Keywords: Long non-coding RNA, Extracellular matrix, Tumor microenvironment
1. Introduction
Traditionally, the protein-coding genes have been investigated as responsible for the pathological manifestation of human diseases. On the contrary, the non-coding component of human genome was considered as “junk-DNA″ or “black matter of DNA”, reflecting the paucity of knowledge and technological approaches able to reveal its involvement in human diseases [1]. The high-throughput technologies available today combined with bioinformatics analyses revealed that nearly 80% of the genome is transcribed, whereas only 2% of the genetic code is translated in protein [2]. These events could be considered as the discovery of a “second genetic code” consisting of non-coding RNAs (ncRNAs) that modified our traditional concept of genomic organization. The abundance of actively transcribed ncRNA indicating their potential pivotal role in homeostatic and pathological mechanisms; this hypothesis has been confirmed by a plethora of emerging evidences in different diseases, especially in cancer [3].
The family of ncRNA is very large and to date we know only a part of these molecules, which are classified in many ways: according to their genomic position, to their mechanism of action or simply based on their size. According to the latter one, the long ncRNAs (lncRNAs; >200 nucleotides) is a newer class of ncRNAs divided into five categories with respect to the nearest protein-coding transcripts: sense, antisense, bidirectional, intronic, and intergenic. Based on their molecular mechanism of action, lncRNA can be divided in four sub-classes: guide, scaffold, signaling and decoy molecules [4]. Guide lncRNAs regulate gene expression by physically directing ribonucleoprotein complexes (RNP) to specific genomic regions. Ribonucleoprotein complexes are in turn composed by scaffold lncRNAs acting as platforms on to which multiple proteins can be assembled in functional units [5]. Signaling lncRNAs are associated with specific signaling pathways. Finally, active transcription factors are sequestered by decoy lncRNAs through direct linkage, reducing their bioavailability. Intriguingly, it has been also demonstrated an interaction between short- (miRNA) and long-non coding RNA. In fact, miRNAs bioavailability can be finely regulated by interacting with specific miRNA response elements (MRE) within individual lncRNA. In this way lncRNA can function as molecular sponges actively competing with miRNAs' target mRNAs [6]. Certain lncRNAs regulate post-transcriptional mRNA processing, and in particular pre-mRNA splicing [7]. Others form RNA-RNA duplexes with mRNAs to facilitate their degradation [8]. Finally, lncRNAs, which contain open reading frames (ORFs), may also be transcribed [9,10].
In parallel, it is largely demonstrated that tumors arise when the tightly controlled systems of cell homeostasis is perturbed by a series of driving genetic mutations. Thanks to decade of studies on cancer cells, ten year ago Hanahan and Weinberg updated the first version of the hallmarks of cancer, by adding the stromal component of tumor tissue among the factors that foster tumor progression [11]. In fact, recent studies demonstrated that as the cancer progresses, the surrounding microenvironment co-evolves into an activated state through continuous paracrine communication. This activate environment, in which occur the collaborative interactions between neoplastic cancer cells and the corrupted supporting stroma, is called tumor microenvironment (TME). Different cellular and non-cellular players composes TME: cancer-associated fibroblast, immune cells, endothelial cells, pericyte and extracellular matrix (ECM) [12]. These micro-environmental changes are observed in nearly all tumor types, including breast, prostate, pancreas, liver, brain, skin and ovary cancers and contribute to both early and late stages of tumor progression. The alterations in the TME are also critical in metastases development process. Because of their contributions to tumorigenesis, stromal cells, ECM and its proteolytic components in the tumors have emerged as new therapeutic targets.
In this review, we synthesize these themes by exploring the functional cross talk between lncRNAs and their involvement in ECM regulation and remodeling within the tumor microenvironment as a fundamental event implicated in carcinogenesis and metastatic progression.
1.1. Extracellular matrix in cancer
In the recent years, many research efforts have been devoted to understand how the cellular component of tumor niche can initiate and promote cancer development [13]. However, recent progresses have also highlighted the importance of non-cellular components of TME, the extracellular matrix [[14], [15], [16], [17], [18]]. ECM has always been considered an amorphous component of the tissue stroma, with the sole role of scaffold upon which tissue are organized, while now it has been demonstrated that ECM is an essential part of the TME that is surprisingly dynamic and versatile and influences fundamental aspects of cell biology [19]. Directly or indirectly, the ECM regulates almost all cellular behaviors and is indispensable for major developmental processes. ECM is a heterogeneous structure, composed of proteins (collagen family is the most abundant), glycosaminoglycans (i.e. chondroitin sulfate, heparin sulfate and hyaluronic acid), proteoglycans (i.e. hyalectans, aggrecan, versican and decorin), and ECM remodeling enzymes (i.e. ADAM, ADAMTS and cathepsin), which is peculiar from organ to organ in composition, ratio and biochemical modifications [20]. A growing number of studies showed that an abnormal ECM dynamic is a hallmark of cancer. For example, tumors often display desmoplasia, and this fibrotic state is characterized by increased deposition, an altered organization and enhanced post-translational modifications of ECM proteins [21]. The desmoplastic reaction is partially characterized by an increased collagen deposition, including collagen I, II, III, V, and IX, during tumor formation [[22], [23], [24]]. Part of the increase in tissue stiffness can be attributed to excess activities of lysyl oxidase (LOX), which cross-links collagen fibers and other ECM components. For instance, in breast cancer the collagen fibers, rather than relaxed and non-oriented, are often highly linearized and either oriented adjacent to the epithelium or projecting perpendicularly into the tissue [18,25].
1.2. LncRNAs and their involvement in ECM components regulation
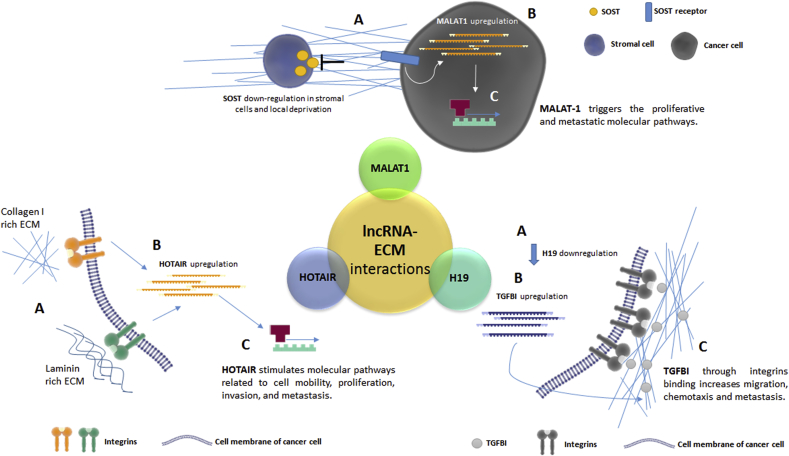
During cancer progression, overcoming the physical barriers by interacting with ECM components is a fundamental event (i.e. the breakthrough of the basal lamina for carcinomas and the penetration of metastatic cells into blood or lymphatic vessel before dispersing to distant organs). Although studies that focus onto lncRNAs role in cancer cell-ECM crosstalk are only in its infancy, some publications highlight their involvement in ECM remodeling and turnover. Fig. 1 summarizes some examples of lnRNA and ECM cross talk in tumor microenvironment.
Fig. 1.
The figure summarizes three molecular mechanisms experimentally demonstrated of interaction between lncRNA and tumor extracellular matrix. Top, the co-cultivation of osteoblasts with prostate cancer cells induce a down-regulation of the secreted glycoprotein SOST (A). Local deprivation of SOST promote an up-regulation of MALAT-1 lncRNA in prostate cancer cell (B), which promote invasion, metastasis and is related to a poor prognosis in different cancer subtypes (C). Right, the down-regulation of H19 lncRNA in metastatic breast cancer cell (A), lead to an increase production of extracellular matrix protein TGFBI (B), which serve as receptor for several integrins promoting adhesion, migration and chemotaxis (C). Left, a stiffer collagen-I/laminin rich ECM (A) as frequent event in cancer progression induce an up-regulation of HOTAIR lncRNA (B), which stimulates invasion pathways in tumor cells (C).
Zhu et al. evidenced that lncRNA H19 has a critical role in prostate cancer progression [26]. H19 lncRNA is expressed in the maternal allele and transcribed from the H19/Igf2 gene cluster located on the chromosome 11. In this study, they found that lncRNA H19 and the H19-derived microRNA-675 were significantly down-regulated in the metastatic prostate cancer cell line M12 compared with the non-metastatic cell line P69. They further demonstrated that the up-regulation of H19 in P69 and PC3 cell lines, significantly increase the expression level of miR-675 causing a repression of cell migration. Interestingly, they found that the expression level of H19 and miR-675 in P69 cells was negatively correlated to the expression of transforming growth factor β induced protein (TGFBI). TGFBI, is an extracellular matrix protein involved in adhesion, migration and chemotaxis by serving as ligand for several integrins [27]. Further mechanistic and functional analysis suggested that the lncRNA H19/miR-675 axis acts as a suppressor of tumor cell migration and prostate cancer metastasis in vitro.
Another interesting proof that lncRNA strictly interact with and contribute to ECM organization and remodeling comes from the paper of Li and colleagues. Paradoxically, they observed a low expression level of the lncRNA HOX transcript antisense RNA (HOTAIR) in invasive claudin-low MDA-MB-231 and Hs578T breast cancer cells line in bidimensional conventional culture. This data seems to be in contrast with other studies that demonstrate HOTAIR over-expression in breast and lung primary and metastatic carcinoma [28,29]. In addition, HOTAIR over-expression correlates with an increased proliferation and resistance to cell death in breast cancer [[30], [31], [32]]. Crucially, in the same study, cultivating the same cell line in a 3-dimensional (3D) culture set-up based of a substrate rich in laminin, they observed a significant up-regulation of HOTAIR. The authors concluded that ECM signaling, mediated by integrin α2 and SRC, may determine the transcription of an alternative HOTAIR isoform, epigenetically-regulated, that stimulates invasion pathways in tumor cells when they are in direct contact with a 3D organized ECM rather than a in conventional bi-dimensional plastic context [33].
The approach of 3D culture model in order to unravel the interplay between HOTAIR lncRNA and ECM components, have been exploited also in the study of Zhuang and colleagues [34,35]. In particular, they explored the interplay between collagen-I and lncRNAs in lung cancer cell carcinogenesis. Collagen-I is enriched in the tumor microenvironment and has a tumor-promoting effect. In this study, they cultivated A549 cells (a human lung adenocarcinoma cell line) and mK-ras-LE cells (a murine lung adenocarcinoma cell line) in a 3D culture model based on a reconstituted basement membrane of matrigel. In this setting, both cell lines undergo acinar morphogenesis. However, cultivating the cell lines in the same set-up with a supplementation of collagen-I, the morphological features of well-differentiated lung adenocarcinoma were disrupted and an over-expression of HOTAIR transcript was observed. On the other hand, collagen-I did not significantly alter the expression of three other lncRNA genes: H19, XIST and MALAT1. In addition, by treating cells with a neutralizing antibody against the collagen-I receptor α2β1 integrin they observed a diminished expression of HOTAIR lncRNA. Finally, they demonstrated that the expression of HOTAIR and collagen-I was concurrently up-regulated in human non-small cell lung cancer.
Cancer cells interact with both ECM proteins and stromal cells which, in turn are able to produce ECM and basement membrane components within the TME. In this landscape, Sebastian and colleagues demonstrated that co-cultivating metastatic prostate cancer cells with osteoblasts, a gene-panel including SOST, a Wnt pathway inhibitor, was down-regulated in osteoblasts [35]. In a further experiment, they demonstrated that SOST knockout in osteoblasts leads to an up-regulation of MALAT-1 lncRNA transcript in prostate cancer cells. Although functional analysis was not performed this suggests that SOST expression may stimulate osteoblasts to promote bone metastasis in prostate cancer cells through MALAT-1 up-regulation. MALAT-1 is one of the first lncRNAs to have proved to have a close involvement in different cancer processes. Furthermore, a series of studies have proposed this lncRNA as a reliable diagnostic and prognostic biomarker, due to its frequent marked up-regulation in various solid tumors type [36].
2. Conclusions
During tumor onset, growth and metastasis, ECM does not remain stable but is dynamically modified by tumor cells and stromal cells, which in turn are influenced by the TME itself. In the recent years, a growing number of studies describes the functional interaction between cancer cells and ECM [37]. Despite this data, many studies continue to focus on tumor cells in isolation, ignoring their context and a big amount of potentially important biological information. Recently, another hidden player involved in tumor-stroma cross-talk has been revealed, the lncRNA. The knowledge of the lncRNAs' role in ECM modulation and remodeling is still lacking, especially if compared to that of microRNAs, but recent findings highlighted that, as microRNAs, the lncRNA deregulation in cancer could be used as diagnostic, prognostic and predictive biomarker. Future advances to improve and extend cancer patients survival will depend on a better understanding of cell genomics and tumor microenvironment crosstalk, in order to facilitate the characterization of a specific tumor identity, so that tailored and individualized medicine will become the rule, and not the exception.
Conflicts of interest
The authors have no conflict of interests to declare.
References
- 1.D'Angelo E., Vicentini C., Agostini M., Kiss A., Baffa R., Scarpa A., Fassan M. MicroRNAs as tools and effectors for patient treatment in gastrointestinal carcinogenesis. Curr. Drug Targets. 2015;16(4):383–392. doi: 10.2174/1389450116666141210091454. [DOI] [PubMed] [Google Scholar]
- 2.Meseure D., Vacher S., Alsibai K.D., Nicolas A., Chemlali W., Caly M., Lidereau R., Pasmant E., Callens C., Bieche I. Expression of ANRIL-polycomb complexes-CDKN2A/B/ARF genes in breast tumors: identification of a two-gene (EZH2/CBX7) signature with independent prognostic value. Mol. Canc. Res. 2016;14(7):623–633. doi: 10.1158/1541-7786.MCR-15-0418. [DOI] [PubMed] [Google Scholar]
- 3.Vicentini C., Fassan M., D'Angelo E., Corbo V., Silvestris N., Nuovo G.J., Scarpa A. Clinical application of MicroRNA testing in neuroendocrine tumors of the gastrointestinal tract. Molecules. 2014;19(2):2458–2468. doi: 10.3390/molecules19022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39(6):925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engreitz J.M., Sirokman K., McDonel P., Shishkin A.A., Surka C., Russell P., Grossman S.R., Chow A.Y., Guttman M., Lander E.S. RNA-RNA interactions enable specific targeting of noncoding RNAs to nascent pre-mRNAs and chromatin sites. Cell. 2014;159(1):188–199. doi: 10.1016/j.cell.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavoff S.A., Mitchell A.J., Schwaid A.G., Cabili M.N., Ma J., Levin J.Z., Karger A.D., Budnik B.A., Rinn J.L., Saghatelian A. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat. Chem. Biol. 2013;9(1) doi: 10.1038/nchembio.1120. 59-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto A., Pasut A., Matsumoto M., Yamashita R., Fung J., Monteleone E., Saghatelian A., Nakayama K.I., Clohessy J.G., Pandolfi P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541(7636) doi: 10.1038/nature21034. 228-+ [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Canc. Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Bhowmick N.A., Neilson E.G., Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternlicht M.D., Lochter A., Sympson C.J., Huey B., Rougler J.P., Gray J.W., Pinkel D., Bissell M.J., Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98(2):137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D. Tensional homeostasis and the malignant phenotype. Canc. Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Erler J.T., Bennewith K.L., Nicolau M., Dornhofer N., Kong C., Le Q.T., Chi J.T.A., Jeffrey S.S., Giaccia A.J. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 17.Erler J.T., Bennewith K.L., Cox T.R., Lang G., Bird D., Koong A., Le Q.T., Giaccia A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Canc. Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levental K.R., Yu H.M., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F.T., Csiszar K., Giaccia A., Weninger W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu G.G., Risteli L., Makinen M., Risteli J., Kauppila A., Stenback F. Immunohistochemical study of type-I collagen and type-I pn-collagen in benign and malignant ovarian neoplasms. Cancer. 1995;75(4):1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::aid-cncr2820750417>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Kauppila S., Stenback F., Risteli J., Jukkola A., Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1998;186(3):262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Huijbers I.J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., Isacke C.M. A role for fibrillar collagen deposition and the collagen internalization receptor Endo180 in glioma invasion. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4 doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu M.J., Chen Q., Liu X., Sun Q., Zhao X., Deng R., Wang Y.L., Huang J., Xu M., Yan J.S. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281(16):3766–3775. doi: 10.1111/febs.12902. [DOI] [PubMed] [Google Scholar]
- 27.Nummela P., Lammi J., Soikkeli J., Saksela O., Laakkonen P., Holtta E. Transforming growth factor beta-induced (TGFBI) is an anti-adhesive protein regulating the invasive growth of melanoma cells. Am. J. Pathol. 2012;180(4):1663–1674. doi: 10.1016/j.ajpath.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–U1148. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E. Functional demarcation of active and silent chromatin domains in human HOX loci by Noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlowska E., Szczepanska J., Blasiak J. The long noncoding RNA HOTAIR in breast cancer: does autophagy play a role? Int. J. Mol. Sci. 2017;18(11) doi: 10.3390/ijms18112317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajjari M., Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015;12(1):1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai B., Song X.Q., Cai J.P., Zhang S. HOTAIR: a cancer-related long non-coding RNA. Neoplasma. 2014;61(4):379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- 33.Li M., Li X., Zhuang Y., Flemington E.K., Lin Z., Shan B. Induction of a novel isoform of the lncRNA HOTAIR in Claudin-low breast cancer cells attached to extracellular matrix. Mol. Oncol. 2017;11(12):1698–1710. doi: 10.1002/1878-0261.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang Y., Wang X., Nguyen H.T., Zhuo Y., Cui X.P., Fewell C., Flemington E.K., Shan B. Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J. Hematol. Oncol. 2013;6 doi: 10.1186/1756-8722-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastian A., Hum N.R., Hudson B.D., Loots G.G. Cancer-osteoblast interaction reduces sost expression in osteoblasts and up-regulates lncRNA MALAT1 in prostate cancer. Microarrays. 2015;4(4):503–519. doi: 10.3390/microarrays4040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto R., Mayeda A., Yoshida M., Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochim. Biophys. Acta. 2016;1859(1):192–199. doi: 10.1016/j.bbagrm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Piccoli M., D'Angelo E., Crotti S., Sensi F., Urbani L., Maghin E., Burns A., De Coppi P., Fassan M., Rugge M. Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research. J. Cell. Physiol. 2018;233(8):5937–5948. doi: 10.1002/jcp.26403. [DOI] [PubMed] [Google Scholar]