Abstract
Adsorptive media technology is frequently used by small water systems to remove arsenic because of its simplicity and efficiency. Current practice is to replace the media when it no longer reduces arsenic below the maximum contaminant level of 10 μg/L that the US Environmental Protection Agency has set for drinking water. Media replacement typically accounts for approximately 80% of the total operational and maintenance costs. One potential option to reduce the cost is onsite regeneration and reuse of the media. To evaluate the regeneration option, three consecutive regeneration studies were conducted on a full-scale adsorptive media system. This article, the first of a two- part series, describes the regeneration process and its efficacy in stripping arsenic and other contaminants from exhausted media. Study results found that a three- step regeneration process of media backwash, caustic regeneration, and acid neutralization conditioning proved effective for stripping arsenic and other contaminants from the exhausted media.
Keywords: adsorptive media, arsenic, drinking water treatment, regeneration
In 2001, the US Environmental Protection Agency (USEPA) lowered the maximum contaminant level (MCL) for arsenic in drinking water from 50 to 10 μg/L (USEPA 2001a). At the time of the revision, the agency estimated that approximately 4,100 water systems would be affected by the revised MCL and gave these systems five years (until Jan. 23, 2006) to come into compliance (USEPA 2001b). USEPA projected that many of the water systems would install a treatment system and identified seven treatment technologies as a best available technology (BAT). The agency also evaluated two other technologies: coagulation-assisted microfiltration and the adsorptive media (AM) granular ferric hydroxide. Although both technologies were determined to be effective for arsenic removal, neither was designated as a BAT because of the lack of published data demonstrating performance for a range of water qualities.
To assist water systems, particularly small systems, in selecting a cost-effective arsenic removal technology, USEPA conducted the Arsenic Demonstration Program (ADP) from 2003 to 2011, which evaluated the performance of 50 full- scale arsenic removal systems (Wang & Chen 2011). Of the 50 systems evaluated, 28 (56%) were AM technologies involving 10 different media products. Three of the media products registered were iron-based—a granular ferric oxide1 (GFO), a granular ferric hydroxide2 (GFH), and a granular ferric oxide/hydroxide3—and were developed in the late 1990s and early 2000s (Sperlich et al. 2008, Mohan & Pittman 2007, Amy et al. 2005, Sperlich et al. 2005, Chang et al. 2004, Thirunavukkarasu et al. 2003, Driehaus et al. 1998). The GFO media was used at half (14) of the 28 AM project sites. The results of the technology demonstration program and other related information have led to the frequent use of AM technology as an arsenic removal method by small water systems, mainly because of its simplicity, small footprint, and effectiveness (Mondal et al. 2013, Jain & Singh 2012, Giles et al. 2011, Wang & Chen 2011, Sabbatini et al. 2009, Cundy et al. 2008, Choong et al. 2007, Mohan & Pittman 2007, Xie et al. 2007, Jang et al. 2006, Westerhoff et al. 2006, Daus et al. 2004).
THE AM PROCESS
Process description and performance.
The AM process consists of packed bed(s) of media that has the ability to adsorb arsenic from water. Eventually, the AM becomes saturated to the point where it no longer has the ability to reduce the arsenic level to below the MCL. When this occurs, current practice is to replace the exhausted media with new virgin media.
Several variables affect the life and performance of the media, including but not limited to empty bed contact time (EBCT) and water quality. For iron-based media, the most significant water quality parameters affecting bed life are the source water pH and the arsenic, phosphate, silicate, and vanadium concentrations. The results of the ADP studies found media bed life to vary widely, from as low as 3,700 bed volumes (BVs) to as high as 100,000 BVs. Because of the lack of extensive performance data, estimating the number of BVs to arsenic breakthrough (10 μg/L) has been an art rather than a science. Estimation of bed life has improved over recent years to the point where some vendors are providing a warranty on media bed life. If the media does not achieve its estimated bed life, the vendor will provide a cost credit for the difference between the estimated and actual life toward the purchase of new media.
Operational cost.
The operational and maintenance (O&M) cost elements of the AM process can be divided into categories of chemicals, electricity, labor, and maintenance. Chemical additions can include an oxidant (e.g., chlorine) for arsenite conversion to arsenate, acid or carbon dioxide for pH reduction, and a caustic solution or air-stripping to increase the pH of the treated water if required. Electricity is generally the least expensive item and typically adds little to the pumping cost that existed before the treatment system was installed. Labor costs reflect the time required to support the operation of the system, including maintenance and hourly labor cost. Maintenance includes the replacement and disposal of exhausted media.
The cost of media replacement can be substantial. The performance and cost data for the 28 AM systems evaluated by the ADP showed that media replacement averaged more than 80% of the total O&M cost and was as high as 95% of the total O&M cost at some sites (Sorg et al. 2015, Wang & Chen 2011). The cost of new media on a dollar-per-cubic-foot basis can range from around $100 to as high as $600. The labor to remove and reload the new media plus the disposal of the exhausted media adds to the total replacement cost. For very small systems, the labor cost can be as much as the media cost. For the systems in the ADP that changed out their media during the performance evaluation study periods, the cost of media replacement (based on $/1,000 gpm of treated water) ranged from $0.58 to as high as $22.05.
The most common way that water systems reduce their media replacement cost is replacement of the existing media with a lower-cost media and/or one that has a longer bed life. While some water systems have found that switching to a different media product has produced some success in lowering their O&M cost, the tactic has not always achieved the desired cost benefit. Another possible option to reduce media replacement cost is on-site regeneration of the exhausted media. On-site regeneration has the potential to lower the O&M cost substantially, but until the current study it had not been attempted on-site on a full-scale system.
Media regeneration.
Most media products are not marketed as being regenerable, with the notable exception of the modified resins products (Möller et al. 2011, 2008; Sylvester et al. 2007). Although these modified resins are capable of being regenerated, the authors of the current study are not aware of these resins being regenerated on-site. In 2008, questions were raised by several state agencies over whether off-site regeneration negated NSF International Standard 61 (NSF/ANSI 2013) certification of the media. These concerns led to an NSF review of the off-site regeneration process and the development of a set of requirements for off-site regenerated media in order to maintain its NSF Standard 61 certification. As would be expected, these requirements (media analyses) increased the cost of off-site media regeneration. For very small systems using small quantities of media, off-site regeneration likely will be cost-prohibitive compared with media replacement. On-site regeneration, however, does not have these requirements, and depending on the quantity of exhausted media and the system size, it may offer an option to lower the O&M cost of the process.
Activated alumina (AA) has a long history of use for fluoride removal with on-site regeneration (Rubel 1984). AA is also effective for arsenic removal, and pilot studies on arsenic removal have shown that the media can be regenerated following approximately the same regeneration process used for fluoride (Ghosh & Gupta 2012, Clifford et al. 2011, Rubel 2003, Hathaway & Rubel 1987, Rubel & Williams 1980). In both cases, the stripping of the fluoride and/or arsenic requires a caustic solution to raise the pH. When AA is regenerated, the primary difference between regeneration of AA for fluoride removal and regeneration of AA for arsenic removal is the strength of the caustic solution: a 1% solution for the former and a 4% solution for the latter (Rubel 2003, Hathaway & Rubel 1987). Because of the relatively high arsenic removal capacity of AA (thousands of BVs because of low concentrations in micrograms per liter), AA can be used on a throwaway basis for arsenic removal. Fluoride occurs in groundwaters in milligrams- per-liter concentrations, and therefore the AA removal capacity is significantly lower (hundreds of BVs), requiring regeneration to be cost-effective.
Focus and objectives of current research
Given that iron-based media and AA behave similarly, the authors of this study proposed that iron-based media products could also be regenerated. Using exhausted iron-based media from several ADP sites, laboratory (jar and column tests) and pilot-study tests were conducted to answer three initial questions. First, could a caustic solution, similar to that used for AA, be used to strip arsenic from these iron-based products? Second, would the physical properties (primarily dissolution) be affected? Third, following regeneration, would the arsenic removal efficacy be similar to that of new media? Other authors have suggested that iron-based media cannot be regenerated because of this potential dissolution problem (Möller et al. 2011, 2008; Sylvester et al. 2007).
Laboratory studies conducted using 1–4% caustic solutions showed that a 4% solution (pH 13) could remove up to 92% of the arsenic from some of the exhausted media products tested, with the GFO media achieving the best results (Chen et al. 2015). Test results also indicated that the 4% caustic solution did not dissolve any iron. Pilot column field tests using the regenerated media showed that the media could achieve arsenic removal somewhat similar to virgin media.
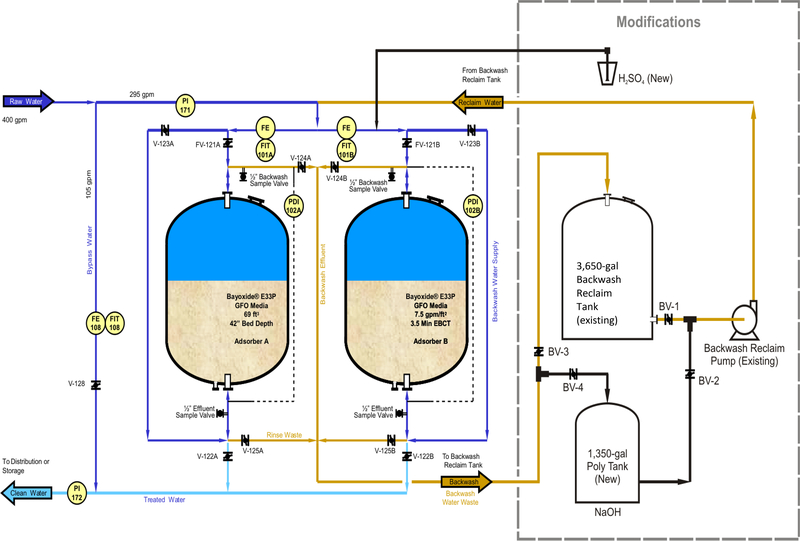
Following the successful results of these laboratory and pilot studies, three full-scale plant studies were undertaken of the regeneration process at the 295-gpm arsenic removal system owned and operated by the Twentynine Palms Water District (TPWD) in California. The three regeneration studies were conducted with technical and financial assistance from USEPA and its contractor, Battelle. The existing AM treatment system was purchased from a supplier4 and consisted of two tanks of the GFO media (Figure 1).
FIGURE 1.
Arsenic removal system with modifications, Twentynine Palms, CA.
The three principal objectives of the regeneration studies were to
determine the effectiveness of the regeneration process to strip arsenic from the exhausted media,
evaluate the subsequent performance of the regenerated media to remove arsenic, and
compare the cost of regeneration with the cost of media replacement.
The second and third regeneration process studies were slightly modified to reduce the amount and the method of disposal of the regenerant wastewater. This article, the first of two parts, describes the regeneration process(es) and the effectiveness of the first three regeneration processes to strip arsenic and other contaminants from the exhausted GFO media.
MATERIALS AND METHODS
Arsenic treatment system.
The arsenic removal system used in this research consists of two 5-ft-diameter adsorption vessels in parallel (tanks A and B), each containing 69 ft3 of GFO pelletized media (see the photograph on page 15). Each vessel was designed for a flow rate of 148 gpm, yielding an EBCT of 3.5 min. The system was installed in September 2007 and treated approximately 48 mil gal (or 46,500 BVs) of water before the arsenic level of the system effluent reached 10 μg/L in October 2008. The source water quality (water samples of Mar. 18, 2009) and system design information are provided in Tables 1 and 2. As shown in Table 1, the total arsenic concentration of the well water was in the range of 17–19 μg/L, existing primarily as soluble arsenate.
Table 1.
Source water quality of the arsenic removal system, Twentynine Palms, CA
| Parameter | Unit | Facility Data(a) | Battelle Data |
|---|---|---|---|
| Date | NA | 6/07 | 03/18/09 |
| pH | S.U. | 7.9 | 8.1 |
| Temperature | °C | 20.0 | NA |
| DO | Mg/L | NA | NA |
| ORP | mV | NA | NA |
| Total Alkalinity (asCaCO3) | mg/L | 100 | 104 |
| Total Hardness (as CaCO3) | mg/L | 42.0 | 45.0 |
| Ca Hardness (as CaCO3) | mg/L | NA | 39.0 |
| Mg Hardness (as CaCO3) | mg/L | NA | 6.0 |
| Turbidity | NTU | ND | NA |
| TDS | mg/L | 160 | 174 |
| TOC | mg/L | NA | <1.0 |
| Nitrate (as N) | mg/L | NA | 2.2 |
| Ammonia (as NH3) | mg/L | NA | <0.05 |
| Chloride | mg/L | NA | 11.0 |
| Fluoride | mg/L | NA | 2.5 |
| Sulfate | mg/L | 14.0 | 13.7 |
| Silica (as SiO2) | mg/L | 25.0 | 22.5 |
| Phosphate (as PO4) | mg/L | 0.1 | NA |
| P (total) (as P) | μg/L | NA | <5 |
| As (total) | μg/L | 19.3 | 17.5 |
| As (soluble) | μg/L | NA | 18.4 |
| As (particulate) | μg/L | NA | <0.1 |
| As(III) | μg/L | 3.3 | 0.2 |
| As(V) | μg/L | NA | 18.2 |
| Fe (total) | μg/L | 50 | 362 |
| Fe (soluble) | μg/L | NA | 49 |
| Mn (total) | μg/L | 5.0 | 8.5 |
| Mn (soluble) | μg/L | NA | 4.7 |
| U (total) | μg/L | NA | 14.7 |
| U (soluble) | μg/L | NA | 14.7 |
| V (total) | μg/L | 5.0 | 7.3 |
| V (soluble) | μg/L | NA | 7.0 |
| Sb (total) | μg/L | NA | 0.1 |
| Se (total) | μg/L | ND | NA |
(a) O&M Manual (STS, June 2007)
TDS=Total Dissolved Solids; TOC= Total Organic Carbon
NA= Not available; ND=Not Detected
Table 2.
Design specifications of the arsenic removal system, Twentynine Palms, CA.
| Parameter | Value |
|---|---|
| Number of Adsorption Vessels | 2 |
| Vessel Size (in) | 60 D × 87 H |
| Vessel Cross Sectional Area (ft2) | 19.6 |
| Type of Media | Bayoxide E33(P)* |
| Media Volume(ft3/vessel) | 69 |
| Media Bed Depth (in) | 42 |
| Design Flowrate (gpm/vessel) | 148 |
| Hydraulic Loading (gpm/ft2) | 7.6 |
| EBCT (min) | 3.5 |
| Average Use Rate (gpd) | 285,260 |
| Flowrate (gpm) | 240 |
| Hydraulic Loading (gpm/ft2) | 12 |
| Duration (min/vessel) | 10 |
| Frequency (time/month) | 1 |
Source: Severn Trent Services
Regeneration plan.
The plan for the first regeneration consisted of replacing one tank of media (tank A) with virgin media, regenerating the second tank of media (tank B), and then evaluating and comparing the performance of the two tanks of media. Before the regeneration of the treatment system could be initiated, the California Department of Public Health (CDPH) required that the water utility submit a regeneration protocol for approval. The protocol that was prepared and submitted to CDPH described the details of the regeneration process and the steps to be taken to place the system back in operation. The regeneration process proposed was based on results of laboratory regeneration tests conducted on several samples of the exhausted media by Battelle in Columbus, Ohio (Chen et al. 2015). After several plan modifications, the regeneration protocol was approved by the state on Mar. 9, 2009, and became an addendum to the utility’s O&M manual for the arsenic removal treatment system.
Because the handling of the wastewater was a secondary issue for the first regeneration (tank B), the plan called for most of the wastewater (75%) to be placed in a 5,000-gal tanker truck and transported to an off-site disposal facility. For the second regeneration of the tank B media, the regeneration process was slightly modified to reduce the quantity of wastewater produced for disposal. Recycling of some of the wastewater was also proposed by the TPWD to further reduce the quantity of wastewater for disposal. The plan consisted of adding ferric chloride to wastewater to precipitate out the arsenic and recycle the supernatant to the front of the plant at very low flows (5–20 gpm); additional details of this activity can be found in the second article of this two- part series (see Sorg et al. 2017 on page 25). Because of California’s rather stringent monitoring requirement, the wastewater was again hauled off-site.
System modifications.
In order to regenerate the media of tank B, several modifications to the existing treatment system were made, including some piping changes and the addition of a 1,350-gal caustic solution holding tank (Figure 1). Before the second regeneration, a second wastewater storage tank (3,650 gal) and a 1,500-gal rinse water holding tank were installed to provide additional on-site wastewater storage capacity as well as the ability to treat the wastewater. These additions brought the total storage capacity to 7,300 gal (not including the 1,350-gal caustic solution tank and the 1,500-gal rinse water holding tank).
Regeneration chemicals.
Two chemicals are used in the regeneration process: sodium hydroxide (NaOH) and sulfuric acid (H2SO4). A 4% caustic solution (800 gal) used to raise the pH of the effluent to 13 was prepared by adding 44 gal of 50% commercial-grade NaOH (NSF listed) to the treated water in the 1,350-gal caustic solution tank. The acid used during a media neutralization step was a commercial-grade 93% H2SO4 (NSF listed) fed directly into the media tank inlet line. Regeneration chemicals were prepared by TPWD employees and research team members wearing protective clothing for safety.
Data and sample collection.
Because the on-site full-scale regeneration test was the first one to take place in California (and to the authors’ knowledge, in the United States), the state required that the very first regeneration process be extensively monitored (primarily for arsenic and pH). In addition, immediate on-site testing of the water quality after regeneration was required before the system could be placed back in service. The pH of a small side stream of the tank influent and effluent was measured continuously with pH probes placed in small flow beakers, and the values were recorded every 2 min. One effluent sample was collected every 15 min and immediately analyzed on-site for arsenic. In addition, grab samples of the effluent were collected approximately every 120 min, preserved with nitric acid, and shipped to the Battelle chemistry laboratory for analysis of arsenic, uranium, phosphorus, and silicon. Data were also collected during the second and third regenerations, but the amount of data collected during the third regeneration was less than that collected during the first and second regenerations.
To determine the amount of arsenic stripped from the media by the regeneration process, two media core samples were collected approximately 12 in. below the media surface before and after each regeneration (total of four) and shipped to the Battelle laboratory for analysis of arsenic and several other metals.
Composite wastewater samples were also collected from the caustic tank, and a tanker truck was used to haul the wastewater off-site for disposal (see the photographs at the top of page 18). These samples were also shipped to the Battelle laboratory to characterize the quality of the wastewater and to use these data to estimate the amount of arsenic and other contaminants stripped from the media.
Chemical analyses.
Two handheld pH meters5 were used to measure the pH of the influent and effluent of tank B. On-site testing for arsenic was accomplished using a test kit6 that had a range of 10–100 μg/L. Samples exceeding the 100-μg/L limit were diluted and re-analyzed immediately. The test kit used is one of several field kits available for arsenic testing and was selected by the research team because of members’ past experience with the kit as well as evaluation test results of two published test kit studies (Spear et al. 2006, USEPA & Battelle 2003).
The grab effluent samples shipped to the Battelle laboratory were analyzed for arsenic, phosphorus, silicon, and uranium following the procedures detailed in a quality assurance/quality control plan produced by Battelle and approved by USEPA (Battelle 2004). The media core samples were collected before and after regeneration and were digested, and the liquid was analyzed for arsenic and other metals by inductively coupled plasma–mass spectrometry (ICP–MS).
Wastewater disposal.
Because the main focus of the first regeneration study was to determine the effectiveness of the regeneration process to strip the arsenic from the exhausted media and to evaluate the performance of the regenerated media, wastewater production and disposal were a secondary concern. With no on-site disposal option, approximately 75% of the wastewater with high concentrations of arsenic was hauled off-site for disposal at an industrial wastewater disposal plant.
For the second regeneration study, the regeneration process was modified to reduce the amount of wastewater produced. In addition, ferric chloride was added to the tanks containing the caustic solution and the acid neutralization conditioning solutions to reduce the arsenic concentration of the two liquid solutions through adsorption onto precipitated iron solids. Ultimately, these solutions and solids were also disposed of at an off-site disposal facility. More details of the handling of wastewaters produced from the second and third regeneration are presented in the second article in this series (Sorg et al. 2017).
REGENERATION PROCESSES AND RESULTS
Tank B first regeneration process.
The first regeneration of the exhausted GFO media in tank B required 7.5 h and took place Mar. 18–19, 2009. The left-hand photograph at the bottom of this page shows the regeneration team and researchers collecting water samples from tank B during the regeneration process. The process consisted of four steps: (1) media backwash— upflow with well water, (2) caustic regeneration— upflow, (3) media rinse—upflow with distribution system water, and (4) acid neutralization conditioning— downflow (single pass/recirculated acid).
Immediately before the start of the caustic wash, tank B was backwashed with well water for 10 min. To strip arsenic from the media, the pH of the filter effluent must reach ≥13 (Chen et al. 2015). As shown in Figure 2, the effluent pH goal of 13 was reached after approximately 30 min (~1 BV) of recirculating the 4% caustic solution upflow at 17 gpm. The caustic wash continued for another 120 min (4 BVs) during which time the concentration of the caustic solution was increased twice with the addition of 3 and 8 gal of the 50% NaOH concentrated solution to maintain the pH above 13. A photograph of regeneration team members preparing the caustic regeneration solution in the storage tank is shown in the right-hand photograph at the bottom of page 18. The concentrations of arsenic, phosphorus, silica, and uranium of the upflow effluent of tank B during the regeneration process is also shown in Figure 2.
FIGUR 2.
Elution of arsenic and other contaminants from the tank B media by the 1st regeneration process.
Following the caustic wash, the media bed was drained directly to a tanker truck and then rinsed upflow with distribution water taken from a nearby fire hydrant at 15 and 34 gpm for 13 and 47 min, respectively. After the rinse, the media was neutralized to pH 9.2 with a sulfuric acid injected flow at 34 gpm followed by recycling approximately 200 gal of acidic water. The neutralization conditioning process lasted 251 min, which included 148 min (4.4 BVs) of a once-through acid-injected flow and 103 min of recycling. A summary of the entire regeneration process is shown in Table 3.
Table 3.
Summary of the three regeneration processes
| 1st Regeneration – Tank B | 2nd Regeneration – Tank B | 1st Regeneration – Tank A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regeneration Step | Solution | Time (min) | Flowrate gpm (gpm/ft2) |
Volume (gal) |
Time (min) | Flowrate gpm (gpm/ft2) |
Volume (gal) |
Time (min) | Flowrate gpm (gpm/ft2) |
Volume (gal) |
| Backwash Upflow | Raw water | 10 | 195 (10) | 1,950 | 10 | 195 (10) | 1,950 | 10 | 195 (10) | 1,950 |
| Caustic Wash Upflow Recirculated |
4% NaOH | 50 | 17 (0.9) | 811 | 120 | 17 (0.9) | 800 | 120 | 17 (0.9) | 1,130 |
| 4.3% NaOH | 30 | 17 (0.9) | ||||||||
| 4.9% NaOH | 70 | 17 (0.9) | ||||||||
| Water Rinse Upflow |
Treated water | 13 | 15 (0.8) | 1,793 | None | |||||
| 47 | 34 (1.7) | |||||||||
| Acid (a) Neutralization/ Conditioning Downflow |
pH 2.4 water | 148 | 34 (1.7) | 5032 | 138 | 34 (1.7) | 4,692 | 218 | 34 (1.7) | 6,170 |
| Acid Neutralization/ Conditioning Downflow Recirculated |
pH = 2.6 to 5.8 (at TP1) |
32 | 34 (1.7) | 83 | 34 (1.7) | |||||
| pH = 5.9 to 3.0 (at TP1) |
30 | 34 (1.7) | ||||||||
| pH = 2.8 to 6.3 (at TP1) |
41 | 34 (1.7) | ||||||||
| Totals | 434 | 9,586 | 351 | 7,442 | 9,250 | |||||
(a) Sulfuric acid feed adjusted into inlet line
Tank B first regeneration results.
At the completion of the regeneration process when the effluent pH reached 9.2, the effluent arsenic was <5 μg/L as determined by the on-site arsenic test kit. The corresponding effluent grab sample analyzed by ICP–MS was 7.2 μg/L, confirming the arsenic concentration was below the MCL of 10 μg/L. After system startup, grab samples were collected of the well water and the effluent from tank A (virgin media) and tank B (regenerated media) daily during the first week of operation after regeneration. All of these samples had arsenic concentrations well below 5 μg/L, indicating that the regeneration process was very effective.
To determine the amount of arsenic stripped from the media by the regeneration process, two media core samples were collected before and after regeneration, and the four samples were analyzed for arsenic and several other metals (Table 4). Before the first regeneration of tank B, arsenic levels of the two exhausted media samples averaged 1,217 μg/g or 0.12% by weight. The two regenerated media samples averaged 207 μg/g (0.02% by weight), indicating a regeneration arsenic removal efficiency of 83%. In addition, this first regeneration process also removed 74% of barium, 64% of phosphorus, 31% of calcium, and 30% of silicon from the exhausted media.
Table 4.
Summary of the media analyses before and after regenerations
| Sample ID | As | Ba | Ca | Fe | Mg | Mn | P | Si |
|---|---|---|---|---|---|---|---|---|
| 1st Regeneration - Tank B Exhausted Media - ug/g | ||||||||
| Sample A | 1,131 | 43.3 | 3,590 | 496,034 | 1,548 | 1,888 | 294 | 6,281 |
| Sample B | 1,302 | 50.3 | 4,191 | 593,040 | 1,754 | 2,183 | 337 | 7,033 |
| Average | 1,217 | 46.8 | 3,891 | 544,537 | 1,651 | 2,036 | 316 | 6,157 |
| 1st Regeneration - Tank B Regenerated Media - ug/g | ||||||||
| Sample A | 207 | 13.0 | 2,796 | 607,294 | 1,500 | 2,119 | 123 | 6,064 |
| Sample B | 207 | 12.0 | 2,695 | 612,250 | 1,478 | 2,121 | 114 | 4,676 |
| Average | 207 | 12.5 | 2,746 | 609,772 | 1,489 | 2,120 | 119 | 5,370 |
| Percent Removed - % | 83 | 74 | 31 | 0 | 10 | 0 | 64 | 30 |
| 2nd Regeneration - Tank B Exhausted Media - ug/g | ||||||||
| Sample A | 2,078 | 47.8 | 3,114 | 663,289 | 1,693 | 2,282 | 362 | 9,035 |
| Sample B | 2,037 | 46.4 | 2,987 | 636,653 | 1,627 | 2,159 | 364 | 8,371 |
| Average | 2,058 | 47.1 | 3,051 | 649,971 | 1,660 | 2,221 | 363 | 8,703 |
| 2nd Regeneration - Tank B Regenerated Media - ug/g | ||||||||
| Sample A | 328 | 7.2 | 852 | 671,680 | 865 | 2,333 | 179 | 4,988 |
| Sample B | 317 | 6.9 | 941 | 691,942 | 880 | 2,316 | 144 | 4,507 |
| Average | 323 | 7.1 | 897 | 681,811 | 873 | 2,325 | 162 | 4,748 |
| Percent Removed - % | 84 | 85 | 71 | 0 | 47 | 0 | 56 | 45 |
| 1st Regeneration - Tank A Exhausted Media - ug/g | ||||||||
| Sample A | 2,320 | 86.0 | 5,647 | 587,027 | 1,914 | 1,883 | 357 | 12,638 |
| Sample B | 2,341 | 88.4 | 5,924 | 576,514 | 1,952 | 2,001 | 366 | 13,032 |
| Average | 2,330 | 87.2 | 5,785 | 581,771 | 1,933 | 1,942 | 362 | 12,835 |
| 1st Regeneration - Tank A Regenerated Media - ug/g | ||||||||
| Sample A | 274 | 14.2 | 1,385 | 637,095 | 871 | 2,046 | 88.4 | 5,844 |
| Sample B | 288 | 14.3 | 1,448 | 632,386 | 864 | 2,168 | 82.9 | 5,902 |
| Average | 281 | 14.2 | 1,417 | 634,741 | 867 | 2,107 | 85.7 | 5,873 |
| Percent Removed - % | 88 | 84 | 76 | 0 | 55 | 0 | 76 | 54 |
A regeneration efficiency percentage was also calculated using the estimated mass (869 g) of arsenic in the wastewater (determined from analyses of the wastewater samples) and comparing it with the estimated mass (1,267 g) of arsenic on the media before regeneration (as determined by analysis of the exhausted media). Using this method to calculate the removal efficiency, an 87% regeneration efficiency was achieved, which was just slightly above the 83% estimated from the media analyses. Therefore, based on the two methods of determining the removal efficiency, the amount of arsenic removed by the regeneration process of the tank B media was found to be just slightly below that achieved in the laboratory tests (93%) conducted on the same media using the same regeneration process (Chen et al. 2015).
The first regeneration of tank B media produced a total of 7,636 gal of wastewater (not including 1,950 gal of initial media backwash water). The wastewater consisted of 811 gal of spent caustic solution, 1,793 gal of rinse wastewater, and 5,032 gal of acid neutralization wastewater (Table 3). Because the primary goal of the first regeneration test was an evaluation of the effectiveness of the regeneration process and the performance of the regenerated media, the handling and disposal of the wastewater were not essential parts of the current study. Because of the lack of an on-site disposal option, 4,542 gal of the wastewater produced was trucked off-site to an industrial wastewater system in Los Angeles, Calif. The 4,542 gal consisted of 811 gal of the caustic solution, 1,793 gal of rinse water, and 1,938 gal of the acid neutralization water. Before being transported for disposal, the wastewater in the tanker truck was neutralized on-site with 10 gal of 93% H2SO4. The remaining 3,094 gal of acid neutralization water produced during the last 1.5 h of the neutralization/conditioning step was discharged to the on-site reclaim storage tank (arsenic concentration of 0.23 mg/L) and then trucked to the TPWD wastewater treatment plant for disposal.
Tank B second regeneration process.
The second regeneration of the tank B media took place approximately 15 months after the first regeneration (70,000 BVs of treated water). Although the effluent arsenic concentration from tank B was only 6.7 μg/L, the blended distribution water (20% raw well water) arsenic concentration was near 10 μg/L, requiring the need to regenerate either tank A or tank B. Because the arsenic level from tank A (new media) was even lower (5 μg/L), the decision was made to conduct a second regeneration of tank B media rather than regenerate the tank A media. This second regeneration was conducted over a two-day period (July 15–16, 2010).
A cost analysis of the first regeneration process for tank B had shown that wastewater disposal accounted for 80% of the total cost or $10,000. Therefore, one of the major goals of the second regeneration process was to reduce the amount of wastewater for disposal. To accomplish this goal, the regeneration process was reduced to a three-step process of media backwash, caustic wash, and acid neutralization conditioning. By eliminating the upflow water rinse step and modifying the acid neutralization conditioning step (Table 3), the quantity of wastewater was reduced by about 2,100 gal. At the end of the caustic wash step, the caustic wash solution in tank B was drained to the caustic holding tank (via a pump), and tank B was filled with distribution water. Because of a weather condition, the acid neutralization/conditioning step was suspended and completed the next day with the wastewater stored in two 3,650-gal holding tanks.
Figure 3 shows the pH and the concentrations of arsenic, phosphorus, silica, and uranium of the effluent of tank B during the second regeneration process. As shown in the figure, most of the contaminants were released from the media during the first 20–30 min of the 120-min caustic wash step.
FIGURE 3.
Elution of arsenic and other contaminants from the Tank B media by the 2st regeneration process.
Tank B second regeneration results.
To determine the amount of arsenic stripped from the media by the second regeneration process, two media core samples were again collected before and after regeneration and analyzed for arsenic and several other metals (Table 4). Before regeneration, the arsenic concentration of the two exhausted media samples averaged 2,058 μg/g (0.2% by weight). The two regenerated media samples averaged 323 μg/g (0.03% by weight), resulting in a regeneration arsenic removal efficiency of 84%. In addition, the regeneration process also removed 85% of barium, 56% of phosphorus, 71% of calcium, and 45% of silicon from the exhausted media.
Tank A first regeneration process and results.
This third regeneration study—the first on tank A media— took place Apr. 12–13, 2011. Virgin media had been placed in tank A in March 2009 and had treated slightly more than 80,000 BVs of water over a two- year period. Arsenic levels from tank A, tank B, and the blended distribution water (20% raw water) were 8.3, 2.3, and 7.4 μg/L, respectively. Although the treatment system could have continued to operate for several more months, the TPWD, which is located in a desert region of California, opted to regenerate the tank A media before the ambient temperature became too hot to conduct the field work.
The tank A regeneration process was similar to the second tank B regeneration process and consisted of only three steps. After the media was backwashed and the tank drained, the upflow caustic recirculation began and lasted for 2 h but with more limited monitoring (four effluent grab samples analyzed for arsenic only). Similar to the first and second regeneration studies conducted on tank B media, the target pH of 13 was achieved within 30 min. At the end of the caustic wash, a diluted sample (1,000×) of the caustic solution from the caustic holding tank was analyzed for arsenic on-site using the on-site test kit. The arsenic concentration was found to be between 300,000 and 400,000 μg/L. The following day, the arsenic test was repeated (with 5,000× dilution), and the arsenic concentration was found to be ~250,000 μg/L, comparable to a sample (245,400 μg/L) measured by ICP–MS at the Battelle laboratory.
Following the pattern of the two completed regeneration studies, the acid neutralization step lasted for approximately 3.5 h, with the arsenic concentration and pH value measured by the end of this step at 25 μg/L (23 μg/L by ICP–MS) and 8.8, respectively. Because the arsenic level after the acid neutralization step was >10 μg/L, the media bed was rinsed with treated water (approximately 500 gal) until the arsenic was reduced to 3.1 μg/L.
The regeneration process of tank A produced a total of 7,300 gal of wastewater that included 1,130 gal of caustic recirculation solution and 6,170 gal of acid neutralization wastewater (Table 3). Once again, the caustic and acid wastewater solutions were hauled off-site for disposal at an industrial wastewater disposal facility.
Media samples collected before and after regeneration were analyzed for total metals (Table 4). Before regeneration, total arsenic concentrations on the spent media averaged 2,330 μg/g (2.33% by weight). After regeneration, the arsenic concentrations on the media were reduced to 281 μg/g (on average) for a regeneration efficiency of 88%. The regeneration process also removed 76% of phosphorus and 54% of silicon from the spent media.
CONCLUSIONS
The three full-scale regeneration studies conducted on the AM used in the TPWD arsenic removal system yielded the following conclusions regarding the effectiveness of the regeneration process:
A three-step regeneration process of media backwash, caustic regeneration, and acid neutralization conditioning proved effective for stripping arsenic and other contaminants from the exhausted GFO media.
A 4% caustic solution that raises the pH of the regenerant solution to pH 13 is capable of stripping around 85% of the arsenic from an exhausted GFO media without any detrimental effects to the media.
With the proper training and experience in the handling of caustic and acid chemicals, water utility personnel can successfully regenerate an AM system.
ACKNOWLEDGMENT
The authors thank Battelle staff member Vivek Lal for assisting with the on-site sampling activities during the first regeneration study and the Battelle chemical laboratory staff for water and media sample analyses.
The authors also acknowledge efforts of the Twentynine Palms (Calif.) Water District regeneration team in helping with all of the efforts and activities involved in the regeneration studies.
ABOUT THE AUTHORS
Protection Agency (USEPA), 26 W. Martin Luther King Dr., Cincinnati, OH 45268 USA; sorg.thomas@epa.gov. His experience encompasses 53 years with federal environmental programs, the last 44 with USEPA’s drinking water research and development program. A special research emphasis for Sorg has been drinking water treatment technology for removal of inorganic and radionuclide contaminants from water supplies, including arsenic removal. In the past 15 years, his research has focused mainly on treatment technology to remove arsenic from drinking water to support the revised arsenic maximum contaminant level; this effort included responsibility for the USEPA Arsenic Removal
Full-Scale Demonstration Program. In 2003, Sorg received the AWWA A.P. Black Research Award. Abraham S.C. Chen is president of ALSA Tech in North Potomac, Md. Lili Wang is an environmental engineer with USEPA in Washington. Raymond Kolisz is general manager of the Twentynine Palms Water District in California.
DISCLAIMER
The US Environmental Protection Agency (USEPA), through its Office of Research and Development, funded, managed, and collaborated in the research described here. This article has been subjected to the agency’s administrative review and has been approved for external publication. Any opinions expressed in this article are those of the authors and do not necessarily reflect the views of USEPA; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
ENDNOTES
Bayoxide® E33, Severn Trent Services, Fort Washington, Pa.
GFH®, Evoqua Water Technologies, Warrendale, Pa.
ARM® 200, Englehard, Iselin, N.J.
Severn Trent Services, Tampa, Fla.
WTW Multi 340i, Xylem Analytics, Beverly, Mass.
Arsenic Quick™ test kit, Industrial Test Systems, Rock Hill, S.C.
REFERENCES
- Amy G; Chen HW; Drizo A; von Gunten U; Brandhuber P; Hund R; Chowdhury Z; et al. , 2005. Adsorbent Treatment Technologies for Arsenic Removal. Water Research Foundation, Denver. [Google Scholar]
- Battelle, 2004. Quality Assurance Project Plan (QAPP) for Evaluation of Arsenic Removal Technology. US Environmental Protection Agency, Cincinnati. [Google Scholar]
- Chang YJ; Kwan P; Norton M; Reiber S; Chowdhury Z; Kommineni S; Amy G; Sinha S; Benjamin M; & Edwards M, 2004. Demonstration of Emerging Technologies for Arsenic Removal Volume 1: Bench Testing. Water Research Foundation, Denver. [Google Scholar]
- Chen ASC; Sorg TJ; & Wang L, 2015. Regeneration of Iron-Based Adsorptive Media Used for Removing Arsenic From Groundwater. Water Research, 77:6:85. [DOI] [PubMed] [Google Scholar]
- Choong TSY; Chuah TG; Robiah Y; Gregory FL; Koay G; & Azni I, 2007. Arsenic Toxicity, Health Hazards and Removal Techniques From Water: An Overview. Desalination, 17:1:139. [Google Scholar]
- Clifford DA; Sorg TJ; & Ghurye GL, 2011. Ion Exchange and Adsorption of Inorganics Contaminants In Water Quality and Treatment. McGraw-Hill, New York. [Google Scholar]
- Cundy AB; Hopkinson L; & Whitby LD, 2008. Use of Iron-Based Technologies in Contaminated Land and Groundwater Remediation: A Review. Science of the Total Environment, 400:8:42. [DOI] [PubMed] [Google Scholar]
- Daus B; Wennrich R; & Weiss H, 2004. Sorption Materials for Arsenic Removal From Water: A Comparative Study. Water Research, 38:12:2948. [DOI] [PubMed] [Google Scholar]
- Driehaus W; Jekel M; & Hildebrandt U, 1998. Granular Ferric Hydroxide—A New Adsorbent for the Removal of Arsenic From Natural Water. Journal of Water Supply: Research and Technology—Aqua, 47:1:30. [Google Scholar]
- Giles DE; Mohapatra M; Issa TB; Anand S; & Singh P, 2011. Ironand Aluminium-Based Adsorption Strategies for Removing Arsenic From Water. Journal of Environment Management, 92:7:3011. [DOI] [PubMed] [Google Scholar]
- Ghosh D & Gupta A, 2012. Economic Justification and Eco-Friendly Approach for Regeneration of Spent Activated Alumina for Arsenic Contaminated Groundwater Treatment. Resources, Conservation and Recycling, 61:1:118. [Google Scholar]
- Hathaway SW & Rubel F Jr., 1987. Removing Arsenic From Drinking Water. Journal AWWA, 79:8:61. [Google Scholar]
- Jain CK & Singh RD, 2012. Technological Options for the Removal of Arsenic With Special Reference to South East Asia. Journal of Environmental Management, 107:5:1. [DOI] [PubMed] [Google Scholar]
- Jang M; Min SH; Kim TH; & Park JK, 2006. Removal of Arsenite and Arsenate Using Hydrous Ferric Oxide Incorporated Into Naturally Occurring Porous Diatomite. Environmental Science & Technology, 40:5:1636. [DOI] [PubMed] [Google Scholar]
- Mohan D & Pittman CU Jr., 2007. Arsenic Removal From Water/ Wastewater Using Adsorbents—A Critical Review. Journal of Hazardous Materials, 142:1–2:1. [DOI] [PubMed] [Google Scholar]
- Möller T; Bagchi D; & Sylvester P, 2011. Field Pilot Evaluations of Iron Oxide-Based Arsenic Adsorption Media. Journal AWWA, 103:1:93. [Google Scholar]
- Möller T & Sylvester P, 2008. Effect of Silica and pH on Arsenic Uptake by Resin/Iron Oxide Hybrid Media. Water Research, 42:6–7:1760. [DOI] [PubMed] [Google Scholar]
- Mondal P; Bhowmick S; Chatterjee D; Figoli A; & Wan der Bruggen B, 2013. Remediation of Inorganic Arsenic in Groundwater for Safe Water Supply: A Critical Assessment of Technological Solutions. Chemosphere, 92:3:157. [DOI] [PubMed] [Google Scholar]
- NSF/ANSI (NSF International/American National Standards Institute), 2013. NFS/ANSI 61: Drinking Water System Components—Health Effects. NSF International, Ann Arbor, Mich. [Google Scholar]
- Rubel F Jr., 2003. Design Manual: Removal of Arsenic From Drinking Water by Adsorptive Media. EPA/600/R-03/019, National Risk Management Research Laboratory, Cincinnati. [Google Scholar]
- Rubel F Jr., 1984. Design Manual: Removal of Fluoride From Drinking Water by Activated Alumina. EPA/600/2–84/134, US Environmental Protection Agency, Washington. [Google Scholar]
- Rubel F Jr. & Williams FS, 1980. Pilot Study of Fluoride and Arsenic Removal From Potable Water. EPA-600/2–80-100, US Environmental Protection Agency, Washington. [Google Scholar]
- Sabbatini P; Rossi F; Thern G; Marajofsky A; & Fidalgo de Cortalezzi MM, 2009. Iron Oxide Adsorbers for Arsenic Removal: A Low-Cost Treatment for Rural Areas and Mobile Applications. Desalination, 248:5:184. [Google Scholar]
- Sorg TJ; Kolisz R; Chen ASC; & Wang L, 2017. Regenerating an Arsenic Removal Iron-Based Adsorptive Media System, Part 2: Performance and Cost. Journal AWWA, 109:5:25 https://doi.org/10.5942/jawwa.2017.109.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg TJ; Wang L; & Chen ASC, 2015. Costs of Small Drinking Water Systems Removing Arsenic From Groundwater. Journal of Water Supply: Research and Technology—AQUA, 64:3:219. [Google Scholar]
- Spear JM; Ahou YM; Cole CA; & Xie YF, 2006. Evaluation of Arsenic Field Test Kits for Drinking Water Analysis. Journal AWWA, 98:12:97. [Google Scholar]
- Sperlich A; Schimmelpfennig S; Baumgarten B; Genz A; Amy G; Worch E; & Jekel M, 2008. Predicting Anion Breakthrough in Granular Ferric Hydroxide (GFH) Adsorption Filters. Water Research, 42:1:2073. [DOI] [PubMed] [Google Scholar]
- Sperlich A; Werner A; Genz A; Amy G; Worch E; & Jekel M, 2005. Breakthrough Behavior of Granular Ferric Hydroxide (GFH) Fixed- Bed Adsorption Filters; Modeling and Experimental Approaches. Water Research, 39:6:1190. [DOI] [PubMed] [Google Scholar]
- Sylvester P; Westerhoff P; Möller T; Badruzzaman M; & Boyd O, 2007. A Hybrid Sorbent Utilizing Nanoparticles of Hydrous Iron Oxide for Arsenic Removal From Drinking Water. Environmental Engineering Science, 24:1:104. [Google Scholar]
- Thirunavukkarasu OS; Viraraghavan T; & Subramanian KS, 2003. Arsenic Removal From Drinking Water Using Granular Ferric Hydroxide. Water SA, 29:2:161. [Google Scholar]
- USEPA (US Environmental Protection Agency) & Battelle, 2003. Environmental Technology Verification Program Report: ETV Joint Verification Statement, Arsenic Test Kit. Battelle, Columbus, Ohio. [Google Scholar]
- USEPA, 2001a. National Primary Drinking Water Regulations; Arsenic and Clarification to Compliance and New Source Contaminant Monitoring. 40 CFR parts 9, 141, 142; Final Rule. Federal Register, 66:14:6076. [Google Scholar]
- USEPA, 2001b. Technical Fact Sheet: Final Rule for Arsenic in Drinking Water. EPA 815-F-00–016, Office of Water, Washington. [Google Scholar]
- Wang L & Chen ASC, 2011. Costs of Arsenic Removal Technologies for Small Water Systems: U.S. EPA Arsenic Removal Technology Demonstration Program. EPA/600/R-11/090, National Risk Management Research Laboratory, Cincinnati. [Google Scholar]
- Westerhoff P; De Haan M; Martindale A; & Badruzzaman M, 2006. Arsenic Adsorptive Media Technology Selection Strategies. Water Quality Research Journal of Canada, 41:2:171. [Google Scholar]
- Xie B; Fan M; Banerjee K; & Van Leeuwen J, 2007. Modeling of Arsenic (V) Adsorption Onto Granular Ferric Hydroxide. Journal AWWA, 99:1:92. [Google Scholar]