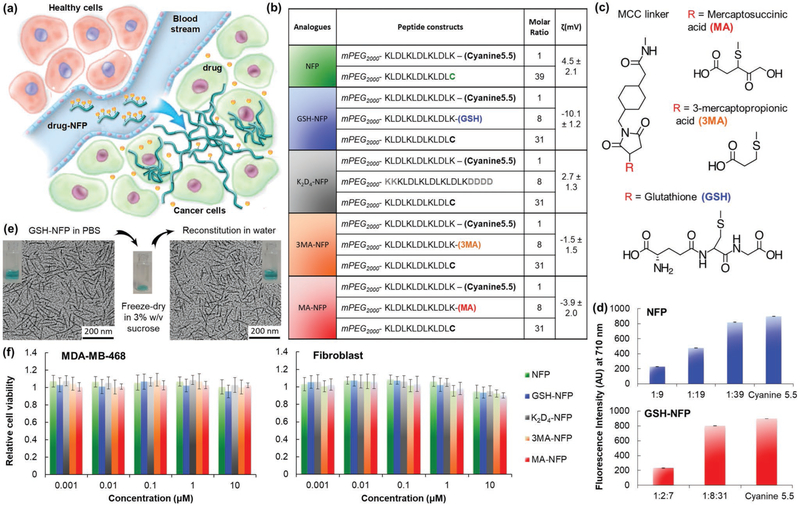
Figure 1.
Design of advanced NFP analogues to enhance tumoral uptake, penetration, and local retention. a) NFP has a high aspect ratio that promotes its uptake by solid tumors. Multiple NFPs can penetrate tumor tissue and subsequently transform into larger interfibril networks via in situ activation by tumor-associated proteases, thus minimizing lymphatic clearance. When used for drug delivery, NFP prolongs the drug-tumor contact time to achieve more effective treatment. b) A table showing the peptide composition and surface charge (zeta potential) of the NFP analogues. The peptide derivatives were used to coassemble the nanofibers. c) Chemical structures of the electron donor glutathione or its derivatives (mercaptosuccinic or 3-mercaptopropionic acids) and the MCC linker used to conjugate them to the core peptide sequence. d) Charts showing the fluorescence intensities of naive NFP (top panel) and GSH-NFP (bottom) (0.1 × 10−6 m of fluorophore content) assembled from different ratios of the peptide constructs (b). At a 1:39 molar ratio of Cyanine5.5 and naked peptide constructs, the nanofibers displayed minimal self-quenching, as shown by the comparable fluorescence intensity to the free Cyanine5.5. e) GSH-NFP could be formulated in powder for long-term storage. Transmission electron microscopic images revealed that the nanofiber (10 × 10−6 m of peptide content) displayed the same size and morphology in sucrose (3% w/v) in a PBS buffer, before freeze-drying into powder or the reconstitution of lyophilized powder with water for injection. f) Graphs showing the cytotoxicity profile of the NFP analogues incubated for 3 days with the human triple-negative breast cancer MDA-MB-468 and fibroblast cell lines. Cell viability was determined using CellTiter Glo reagent (Promega), according to the manufacturer’s instructions.