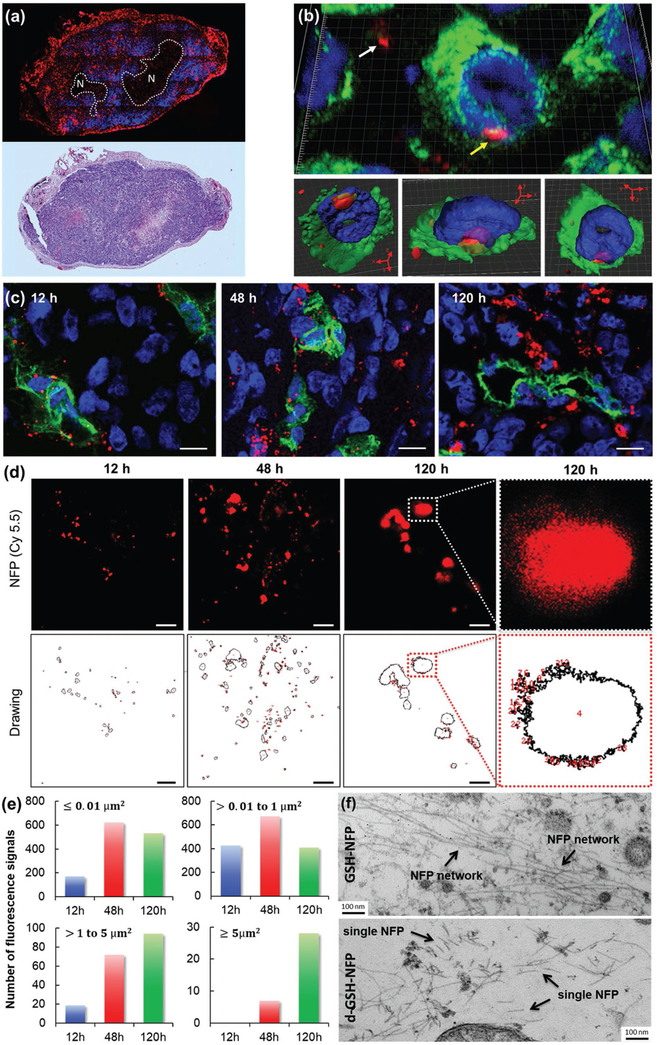
Figure 3.
GSH-NFP displayed tumor penetration, infiltration, and invasion properties. a) Histologic studies showed that the GSH-NFP (red) had invaded the tumor 48 h after a single IV injection of GSH-NFP. The representative tumor section was stained with DAPI (blue) prior to fluorescence imaging. H&E staining was also performed on the adjacent tumor section to confirm the necrotic region (N). b) Confocal microscopic images (using Panoramic Confocal) of GSH-NFP (red) in tumor tissue 48 h post injection. The yellow arrow indicates nanofibers within cancer cells, whereas the white arrow indicates nanofibers in the interstices between the cells. DAPI (blue) and FITC-labeled anti-vimentin antibody (green) were used to stain the nucleus and the cytoskeleton, respectively. The same cell image was processed as a density map and observed in different projection views (bottom panel). c) Confocal microscopic images (using Leica TCS SP8 STED) of the tumor sections collected 12, 48, and 120 h after IV injection of GSH-NFP (5 nmol of fluorophore content). The nanofibers (Cy5.5 fluorescence channel) were imaged using STED 775 nm depletion laser. The blood vessels and cellular nucleus were stained with CD31 (green) and DAPI (blue), respectively. The scale bar represents 10 μm. The image resolution is 0.2 × 0.2 μm2 per pixel. d) Representative high-resolution (35 × 35 nm2 per pixel) STED images of the nanofiber structures (Cy5.5 fluorescence channel) in tumors, and their corresponding computer-generated drawings for measuring the number and size of the fluorescence using ImageJ Software. The scale bar represents 5 μm. e) A plot of the number and size of the fluorescence in the tumor sections at different time points. The images were first acquired using a high-resolution (35 × 35 nm2 per pixel) STED microscope, which were then analyzed for the size and number using ImageJ software. f) TEM analysis of the tumor sections 48 h after IV injection of GSH-NFP or d-GSH-NFP.