Abstract
The ability of herpes simplex virus 1 (HSV-1) to replicate efficiently in differentiated cells is regulated by cellular factors that stimulate viral gene expression, cell survival, and viral morphogenesis. Activation of the canonical Wnt signaling pathway generally increases β-catenin protein levels, cell survival, and growth in dividing cells suggesting this important signaling pathway regulates productive infection. In this study, we demonstrated that a β-catenin specific small molecule inhibitor (iCRT14) reduced HSV-1 titers approximately 10-fold in primary human lung fibroblasts and Vero cells. Furthermore, β-catenin dependent transcription was increased at late times after infection and as expected iCRT14 reduced β-catenin dependent transcription. Although HSV-1 infection increased β-catenin steady state protein levels approximately 4-fold in Vero cells, there was only a nominal increase in human lung fibroblasts. We hypothesized that VP16 regulates β-catenin dependent transcription because VP16 is a viral regulatory protein expressed at late times after infection. In the absence of other viral proteins, VP16 increased β-catenin dependent transcription and β-catenin steady state protein levels. Collectively, these studies suggested the cellular transcription factor β-catenin stimulates productive infection, in part because VP16 enhances β-catenin dependent transcription.
Keywords: HSV-1, Productive infection, β-catenin dependent transcription, β-catenin inhibitor (iCRT14), VP16
1. Introduction
Frequent and serious recurrent eye infections occur in greater than 400,000 individuals (Pavan-Langston, 2000; Smith et al., 1980) followinginfection with herpes simplex virus 1 (HSV-1). Herpes stromal keratitis (HSK) can occur during acute infection or reactivation from latency. Tissue destruction, edema, opacification, corneal scarring, and neovascularization are common symptoms of patients suffering from HSK. Viral-mediated activation of the host encoded heparanase is crucial for viral entry and inflammatory disorders that frequently occur after HSV-1 infection (Agelidis et al., 2015). Following entry of HSV-1 into a permissive cell, viral gene expression in productively infected cells is temporally regulated in three distinct phases: immediate early (IE), early (E), or late (L). A viral tegument component, VP16 binds to two cellular transcription factors, Oct-1 and host cell factor-1 (HCF-1). This transcription complex specifically stimulates IE promoter activity by binding to an enhancer core containing a TAATGARAT motif that is present in all IE promoters (Kristie et al., 1995; Kristie and Roizman, 1988; O’Hare and Goding, 1988; O’Hare and Hayward, 1985).
Wnt family members are secreted glycoproteins that interact with Frizzled receptors and a co-receptor LRP5/LRP6, reviewed in (Clevers and Nusse, 2012). Upon Wnt binding to its receptor, the transcription factor β-catenin is stabilized and enters the nucleus where it interacts with TCF (T-cell factor) family members, reviewed in (Bush et al., 2013; Clevers and Nusse, 2012). Binding of β-catenin to TCF displaces bound co-repressors and recruits co-activators to stimulate expression of Wnt target genes (Alves-Guerra et al., 2007). The Wnt/β-catenin signaling pathway is required for cell proliferation, cell survival, embryogenesis and adult homeostasis in dividing cells (Clevers and Nusse, 2012; Polakis et al., 2012). We recently discovered that bovine herpesvirus 1 (BoHV-1) regulates the Wnt/β-catenin signaling pathway in latently infected sensory neurons (Liu et al., 2016; Workman et al., 2018; Zhu et al., 2017a). These studies suggest that the ability of BoHV-1 to regulate the β-catenin signaling pathway during latency is important because this pathway promotes axonal growth, navigation of axons to their synaptic targets, and neuronal survival, reviewed in (Bamji et al., 2006; Bhardwaji et al., 2013; Murase et al., 2002; Purro et al., 2014; Salinas, 2012). Inhibiting the Wnt/β-catenin signaling pathway reduces BoHV-1 virus yields during productive infection (Zhu and Jones, 2017b). An active Wnt/β-catenin signaling pathway was also reported to stimulate human cytomegalovirus and herpes simplex virus 1 (HSV-1) replication (Choi et al., 2013; Kapoor et al., 2013).
In this study, new studies demonstrate that treatment with iCRT14, a β-catenin specific inhibitor, reduced HSV-1 replication and β-catenin dependent transcription following infection of primary human lung fibroblasts or Vero cells. In Vero cells, HSV-1 increased β-catenin steady state protein levels at late times after infection. Additional studies revealed that VP16 (HSV-1 and BoHV-1) stimulated β-catenin levels in transfected cells, and increased β-catenin dependent transcription.
2. Materials and methods
2.1. Cells and cell viability assay
Human lung fibroblasts (HLF) (ATCC® CCL-201™), Vero cells (ATCC; CCL-81) and Murine neuroblastoma (Neuro-2A) cells (ATCC, CCL-131) were grown in minimal essential medium (MEM) supplemented with 10% FCS, penicillin (10 U/ml), and streptomycin (100 ug/ml). To assess cell viability, cells were treated with iCRT14 (10, 20 or 40 μM for 24 h) and then ATP levels in the cultures measured using the CellTiter-Glo Luminescent Cell Viability Assay (Promega; G7572) according to the manufacturer’s instruction.
2.2. Viruses & plasmids
The McKrae strain of HSV-1 was obtained from Dr. Steve Wechsler. Super 8x TOPflash (Addgene plasmid 12,456) and FOPflash (Addgene plasmid 12,457) were developed in the laboratory of Dr. Randall Moon (Veeman et al., 2003) and were obtained from Addgene. GenScript (Piscataway, NJ) synthesized plasmids that express a myc tagged BoHV-1 VP16 or HSV-1 VP16. The human β-catenin expression construct (S33Y), which expresses a constitutively active Flag-tagged β-catenin protein, was a gift from Bert Vogelstein (Addgene plasmid number 16,519). The mouse non-Flag-tagged β-catenin expression construct (pcDNA3.1/nV5-DEST-beta catenin), which expresses a wild-type protein, was a gift from Valeri Vasioukhin and obtained from Addgene (plasmid number 20,140). For both constructs, β-catenin expression was controlled by the human CMV IE promoter/enhancer. The pcDNA3-FLAG-Luciferease (Addgene 58,792), which contains the human cytomegalovirus (CMV) IE promoter upstream of the luciferase gene, is referred to a CMV-Luc and was a generous gift from Dr. H. Ariga.
2.3. Western blot analysis
Western blots were performed as previously described (Liu et al., 2016) using the following antibodies. A β-catenin monoclonal antibody from Abcam (ab32572) was diluted 1:2000. A HSV-1 specific VP16 antibody (Abcam, ab110226) was diluted 1:1000. A BoHV-1 VP16-specific rabbit polyclonal antibody was obtained from Dr. Vikram Misra (University of Saskatchewan, Saskatoon, CA) and was diluted (1:2000). The mutant S33Y protein was detected using a mouse anti-Flag antibody (Sigma F1804) that was diluted 1:1000. A tubulin antibody was obtained from Dr. Yilin Liu (Licor Biotechnology, Lincoln, NE) and was diluted 1:3000. For all antibodies used for Western blots in this study, the same dilutions of a given antibody was used.
2.4. Dual-luciferase reporter assay
To detect the effect of HSV-1 infection on β-catenin dependent transcription, HFL, Vero, or Neuro-2 A cells were cotransfected with the Super 8x TOPflash plasmid and a plasmid encoding Renilla luciferase under the control of a minimal herpesvirus promoter (Promega) using Lipofectamine® 3000 Transfection Reagent (Invitrogen, L3000075). Higher concentrations of DNA were used for transfecting HFL cells because they are more difficult to transfect than Vero or Neuro-2 A cells. At 36 h posttranfection, cells were infected with HSV-1 at the indicated MOI. At the designated times after infection, cells were harvested and protein extracts subjected to a dual-luciferase assay using a commercially available kit (Promega, E1910) according to the manufacturer's instructions. Luminescence was measured with a GloMax 20/20 luminometer (Promega, E5331). To examine the effect of VP16 on β-catenin dependent transcription, transient transfection studies were performed as previously described (Zhu et al., 2017a; Zhu and Jones, 2017b).
3. Results
3.1. The β-catenin inhibitor, iCRT14, inhibits productive infection
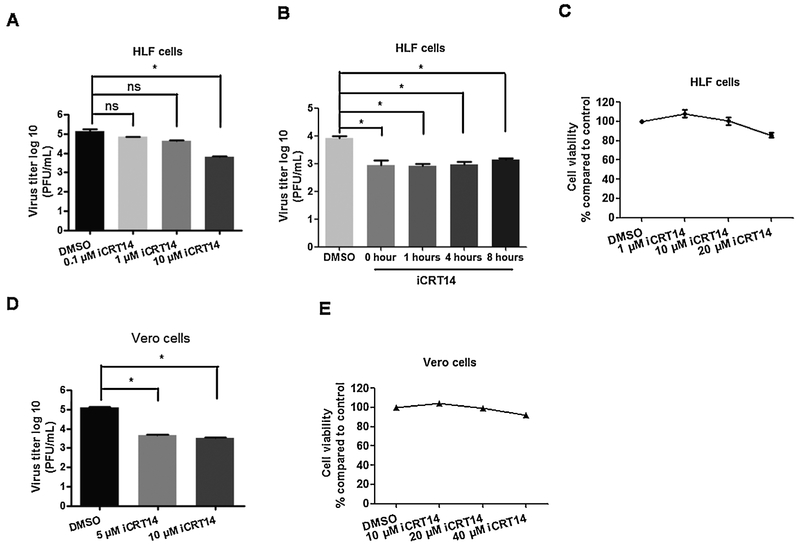
Initial studies examined the effects of the canonical Wnt/β-catenin signaling pathway because this pathway enhances cell growth and survival (Nusse and Clever, 2017; Polakis et al., 2012). This pathway is complicated because in humans there are 19 Wnt family members, 10 Frizzled (Fzd) receptors, and 2 low-density lipoprotein receptor-related protein (LRP) co-receptors (Clevers and Nusse, 2012; Hayward et al., 2008; Nusse and Clever, 2017). Furthermore, a multi-protein β-catenin destruction complex is located in the cytoplasm. A number of Wnt antagonists also exist that prevent Wnt from binding to its receptors (Kawanon and Kypta, 2003; Zorn, 2001). Finally, it is well established that members of the Wnt signaling pathway are expressed in certain cells types, but not others. Regardless of which Wnt signaling proteins are expressed in a given cell, increased β-catenin levels in the nucleus correlates with increased β-catenin dependent transcription, and hence can be used to measure how active the Wnt/β-catenin signaling pathway. Consequently, we tested the effects that a β-catenin specific inhibitor (iCRT14) has on HSV-1 infection of primary human lung fibroblasts (HLF) and established monkey kidney cells (Vero). The interaction between β-catenin and TCF family members is blocked by iCRT14 (Gonsalves et al., 2011) and is a reliable method to examine the effects of β-catenin on productive infection. This small molecule (iCRT14) exhibits specific effects on the Wnt pathway because it is only toxic to human tumor biopsy cultures and colon cancer cell lines that have deregulated Wnt signaling pathways resulting in increased β-catenin protein levels (Gonsalves et al., 2011). In HLF cells, 10 uM iCRT14 reduced virus production by approximately 10-fold (Fig. 1A). Since iCRT14 was added prior to infection, studies were also performed to test what effect it has on productive infection at different times after infection. When 10 uM iCRT14 was added at 0, 1, 2, 4, or 8 h after infection, a ten-fold less virus production was observed (Fig. 1B). Ten uM iCRT14 had little to no toxic effects on HLF cells, as judged by measuring intracellular ATP levels (Fig. 1C) suggesting reduction of viral infection was not due to cell toxicity.
Fig. 1. HSV-1 productive infection is reduced by the β-catenin inhibitor iCRT14.
HLF cells (Panels A) or Vero cells (Panel D) were grown in 6-well dishes and then treated with the designated concentrations of iCRT14 for 2 h prior to infection with HSV-1. Cultures were then infected with HSV-1 at a MOI of 1 for 1 h in the presence of iCRT14 or DMSO as a vehicle control. HLF cells were treated with 10 uM iCRT14 at 0 h, 1 h, 4 h or 8 h after infection (Panel B). After extensive washing with PBS, fresh medium containing the designated concentration of iCRT14 or DMSO control was added to cells. Total virus production in the respective cultures was measured by plaque assay at 24 h after infection. These studies were repeated 3 times and an asterisk denotes significant differences between the DMSO control and cultures treated with iCRT14 (* : P < 0.05) as determined by the Student t-test. The designated iCRT14 concentrations were incubated with uninfected HLF cells (Panel C) and Vero cells (Panel E) for 24 h. ATP levels in the respective cultures were measured to assess cell viability using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega; G7572), according to the manufacturer's instruction.
Relative to the DMSO control, 5 and 10 uM iCRT14 reduced virus production by approximately 10-fold in Vero cells (Fig. 1D). Furthermore, 10 uM iCRT14 had little to no toxic effects on Vero cells (Fig. 1E). Approximately 90% of cells were viable even when cultures were treated with 20 or 40 uM iCRT14 concentrations for 24 h. These studies demonstrated that a β-catenin specific inhibitor, iCRT14, reduced the efficiency of virus production in HLF and Vero cells. Furthermore, studies in HLF cells suggested iCRT14 affected late stages of productive infection.
3.2. Analysis of β-catenin dependent transcription during productive infection
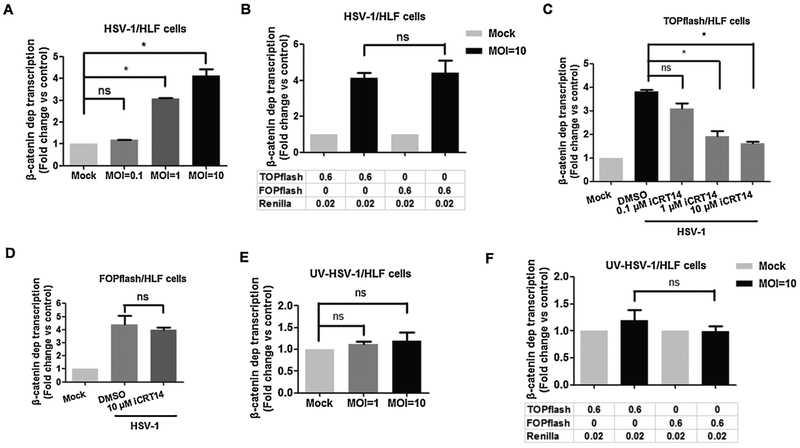
To test whether infection regulated β-catenin dependent transcription, HLF cells were transfected with Super 8x TOPflash and promoter activity measured at different times after infection. When β-catenin is localized to the nucleus, it interacts with a TCF family member bound to a consensus site (5′-T/A-T/A-CAAAG-3′), which culminates in transcriptional activation (Biechele et al., 2012; Bush et al., 2013; Clevers and Nusse, 2012). The plasmid Super 8x TOPflash contains 7 TCF binding sites upstream of a minimal promoter that drives firefly luciferase reporter expression and thus accurately measures β-catenin dependent transcription. At an MO1 of 1, we observed a 3-fold increase in TOPFlash dependent transcription and a 4-fold increase when HLF cells were infected with a MOI of 10 (Fig. 2A). Additional studies examined FOPflash promoter activity 16 h after infection because this construct contains 6 mutated TCF binding sites upstream of the minimal promoter and thus is not transactivated by β-catenin. Although a 4-fold induction of FOPflash dependent transcription was observed during productive infection (Fig. 2B), this may be due to the fact that the minimal HSV-1 thymidine kinase promoter is present in TOPflash and FOPflash. Consequently, viral encoded transcriptional activators may have stimulated FOPflash dependent promoter activity in a β-catenin independent manner. Evidence supporting this prediction comes from the finding that iCRT14 significantly reduced TOPflash dependent promoter activity after infection (Fig. 2C), but had no effect on FOPflash promoter activity (Fig. 2D). When virus preparations were UV-treated, β-catenin dependent transcription (Fig. 2E) or FOPflash dependent transcription (Fig. 2F) was not significantly increased.
Fig. 2. β-catenin-dependent transcription is increased at late times after HLF cells were infection with HSV-1.
HLF cells in 12-well plates were transfected with 0.6 μg of TOPflash or FOPflash luciferase reporter construct and 0.02 μg of the Renilla reporter construct. Renilla reporter construct was used as an internal control to allow normalization of promoter activity. At 36 h after transfection, cells were infected with HSV-1 (Panels A-D) or UV-inactivated HSV-1 (Panels E and F) at the indicated MOI. At 16 h after infection, dual luciferase activity was measured. Where indicated (Panels D and E), the designated concentration of iCRT14 was added immediately after infection. At 16 h after infection, dual luciferase activity was measured. These results are the average of three independent experiments. An asterisk denotes significant differences (P < 0.05) in promoter activity compared to the indicated control by the Student t-test.
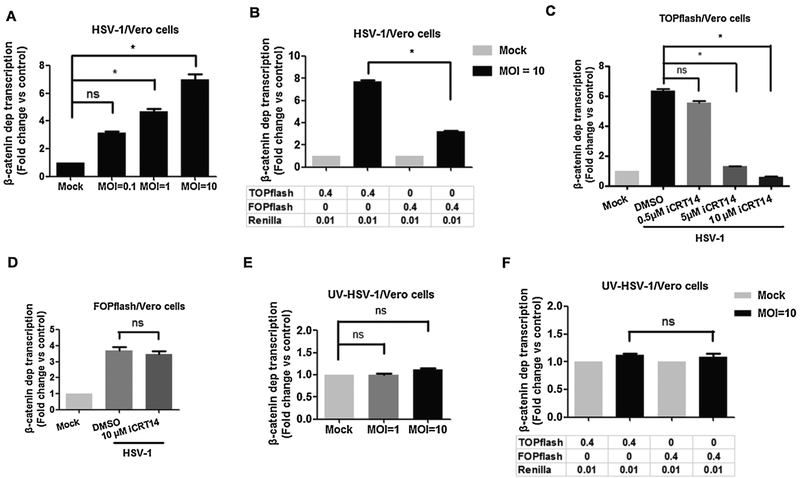
In Vero cells, β-catenin dependent transcription increased more than 4 fold at 16 h after infection when cells were infected with a MOI of 1 and 6 fold when Vero cells were infected with a MOI of 10 (Fig. 3A). At a MOI of 10, TOPflash dependent promoter activity was significantly higher than FOPflash promoter activity at 16 h after infection (Fig. 3B). Although FOPflash promoter activity was approximately 3-fold higher at 16 h after infection; it was significantly less than TOPflash promoter activity. At earlier times after infection, β-catenin dependent transcription was not higher than 16 h after infection (data not shown). Super 8x TOPflash promoter activity at 16 h after infection decreased in a dose-dependent manner when infected cultures were treated with iCRT14 (Fig. 3C). Conversely, 10 uM iCRT14 had no effect on FOPflash promoter activity in Vero cells infected with HSV-1 (Fig. 3D). When Vero cells were incubated with UV-inactivated virus, TOPflash dependent promoter activity (Fig. 3E) or FOPflash dependent promoter activity (Fig. 3F) was not significantly increased. In summary, these studies demonstrated that infection of HLF or Vero cells significantly increased β-catenin dependent transcription at late times after infection. The effect in HLF cells was not as dramatic, in part because FOPflash promoter activity was also increased.
Fig. 3. β-catenin-dependent transcription was increased when Vero cells were infected with HSV-1.
Vero cells were grown in 12-well dishes and then transfected with 0.4 μg of TOPflash or FOPflash luciferase reporter construct and 0.01 μg of the Renilla reporter construct. Renilla reporter construct was used as an internal control to allow normalization of promoter activity. At 36 h after transfection, cells were infected with HSV-1 (Panels A and B) or UV-inactivated HSV-1 (Panels E and F) using the indicated MOI. At 16 h after infection, dual luciferase activity was measured.
Vero cells seeded in a 12-well plate were transfected with 0.4 μg of TOPflash (Panel C) or FOPflash (Panel D) luciferase reporter construct and 0.01 μg of the Renilla reporter construct. Renilla reporter construct was used as an internal control to allow normalization of promoter activity. At 36 h after transfection, cells were infected with HSV-1 at a MOI of 10 and treated with the designated concentration of iCRT14. At 16 h after infection, dual luciferase activity was measured. The results in all panels are the average of three independent experiments. An asterisk denotes significant differences (P < 0.05) in promoter activity compared to the indicated control by the Student t-test.
3.3. Analysis of β-catenin protein expression during productive infection
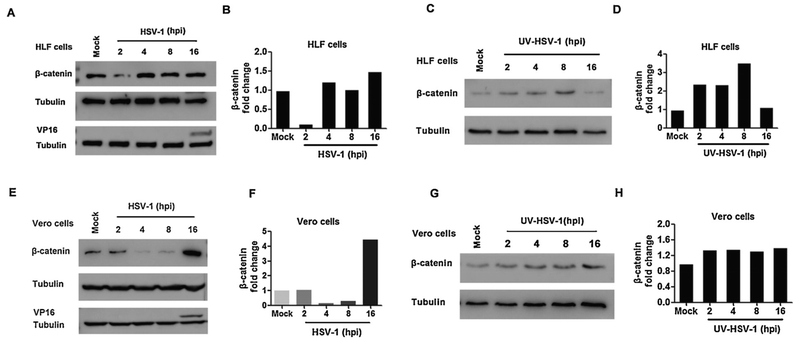
Additional studies examined steady state β-catenin protein levels following infection of HLF and Vero cells. Sixteen hours after HLF cells were infected with a MOI of 1, a modest 1.5 fold increase of β-catenin steady state protein levels was detected (Fig. 4A and B). Conversely, β-catenin protein levels were reduced at 2 h after infection. The late viral protein VP16 was readily detected at 16 h after infection and could be detected at 8 h after infection when the blot was over-exposed (data not shown). Surprisingly, β-catenin steady state protein levels were increased approximately 3-fold when HLF cells were incubated with UV-inactivated virus (Fig. 4C and D). However, β-catenin steady state protein levels were the same as mock-infected cells at 16 h after infection.
Fig. 4. β-catenin steady state protein levels were increased at late stages of productive infection.
HLF cells were infected with HSV-1 (Panels A and B) or UV-inactivated HSV-1 (Panels C and D) at a MOI of 1. Vero cells were infected with HSV-1 (Panel E and F) or UV-inactivated HSV-1 (Panel G and H) at a MOI of 1. Mock-infected cells were included as a control. At the indicated times after infection, total cell lysate was prepared and Western blots performed using specific antibodies that recognize β-catenin, VP16, and Tubulin as described in the materials and methods.
Panel B, D, F and H: The band intensity of β-catenin and Tubulin was quantified with software Image J. Levels of β-catenin were compared to Tubulin and the changes in β-catenin expression are presented. These results are representative of three independent studies.
β-catenin protein levels were increased approximately 4-fold at 16 h after Vero cells were infected (Fig. 4E and F). Conversely, steady state β-catenin protein levels were reduced at 2 and 8 h after infection relative to mock-infected cells. VP16 was readily detected at 16 h after infection and at 8 h after infection when the blot was over-exposed (data not shown). UV-inactivated virus had little effect on β-catenin protein levels in Vero cells (Fig. 4G and H). In summary, these studies suggested that cell-type specific factors played a role in regulating β-catenin protein levels during productive infection. These studies further indicated that in Vero and HFL cells β-catenin protein levels were reduced at early stages of productive infection.
3.4. VP16 stimulates β-catenin dependent transcription in transfected cells
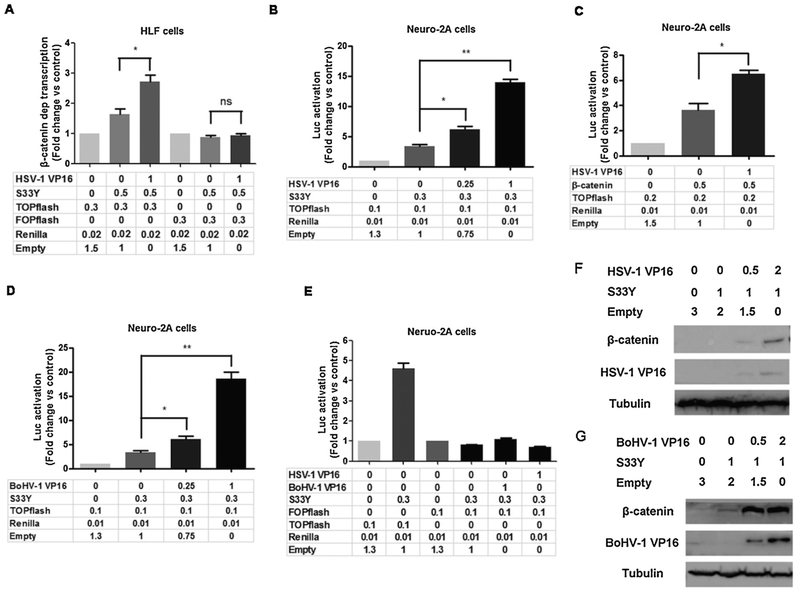
We tested whether VP16 has the potential to regulate β-catenin dependent transcription because VP16 is a crucial viral transcriptional protein that is abundantly expressed at late times after infection (Lemaster and Roizman, 1979; Marsden et al., 1987; McLean et al., 1990). Initial studies tested whether VP16 had effects on a constitutively active β-catenin protein (S33Y). The rational for using a construct that expresses the S33Y protein is this protein is not rapidly degraded by the β-catenin destruction complex, which is typically active in normal cells, for example HLF. In HLF cells, VP16 cooperated with the protein encoded by the S33Y expression plasmid to stimulate TOPflash promoter activity 3-fold (Fig. 5A). In contrast, VP16 and the S33Y construct were unable to activate FOPflash promoter activity.
Fig. 5. VP16 stimulates β-catenin-dependent transcription.
Panel A: HLF cells were cotransfected with 0.3 μg of the TOPflash or FOPflash luciferase reporter, and 0.02 μg of Renilla reporter construct. Where indicated, 0.5 μg of β-catenin (S33Y) and 1 μg of HSV-1 VP16 construct were used to examine the effects that VP16 have on β-catenin dependent transcription in HLF cells. Neuro-2 A cells were cotransfected with 0.1 μg of the TOPflash luciferase reporter and 0.01 μg of Renilla reporter construct. Where indicated, 0.3 μg of activated β-catenin (S33Y) (Panel B) or wild type β-catenin (Panel C) and 1 μg of VP16 at the designated concentration was used to examine the effects that VP16 had on β-catenin dependent transcription in Neuro-2 A cells.
Panel D: Neuro-2 A cells were cotransfected with 0.1 μg of the TOPflash luciferase reporter and 0.01 μg of Renilla. Where indicated, 0.3 μg of S33Y and 1 μg of BoHV-1 encoded VP16 at the designated amount were used to examine the effects that VP16 had on β-catenin dependent transcription in Neuro-2 A cells.
Panel E: Neuro-2 A cells were cotransfected with 0.1 μg of the TOPflash luciferase reporter or the FOPflash plasmid and 0.3 μg of β-catenin (S33Y) plus 1 μg of VP16 (HSV-1 or BoHV-1).
Panels A-E: Results for cells transfected with the TOPflash luciferase reporter and Renilla luciferase under control of a minimal herpesvirus TK promoter were normalized to a value of 1, and fold activation was calculated for other samples. The results are the average of three independent experiments, and error bars denote standard errors. An asterisk denotes significant differences (P < 0.05) in promoter activity compared to the indicated control by the Student t-test.
Neuro-2 A cells in 60 mm dishes were transfected with β-catenin expression plasmid (S33Y) plus VP16 of HSV-1 (Panel F) or BoHV-1 (Panel G) at the designated concentration, β-catenin protein levels in whole cell lysate of transfected Neuro-2 A cells were examined at 48 h after transfection using the Flag-tagged antibody as described in the materials and methods. VP16 protein levels were detected using the antibody described in the materials and methods. The results are representative of three independent experiments.
As a comparison to the results in HLF cells, we examined mouse neuroblastoma cells (Neuro-2 A) because they are easy to transfect, they have low endogenous levels of β-catenin, and β-catenin was expressed at higher levels during late stages of productive infection (data not shown). Neuro-2 A cells are also attractive for HSV-1 studies because it is well established that infection of neuronal cells are crucial for life-long latent infection, Neuro-2 A cells are neuronal like, and can be differentiated into dopamine neurons (Tremblay et al., 2010). Thus, Neuro-2 A cells can be used as a model to test whether VP16 has neuronal specific effects on β-catenin dependent transcription. VP16 consistently cooperated with S33Y construct to significantly stimulate TOPflash promoter activity in a dose-dependent manner (Fig. 5B). A plasmid that expresses wt β-catenin cooperated with VP16 to significantly increase TOPflash promoter activity (Fig. 5C). Relative to S33Y, wt β-catenin was not as active, which was expected because the S33Y protein is resistant to proteolysis. The bovine herpesvirus 1 (BoHV-1) encoded VP16 also cooperated with S33Y to stimulate TOPflash promoter activity in a dose-dependent manner (Fig. 5D). As expected, HSV-1 and BoHV-1 encoded VP16 had little or no effect on FOPflash promoter activity in transfected Neuro-2 A cells (Fig. 5E). HSV-1 (Fig. 5F) or BoHV-1 (Fig. 5G) encoded VP16 consistently increased β-catenin steady state protein levels in transfected Neuro-2 A cells. Although confocal microscopy suggested there was partial co-localization of VP16 with β-catenin in Neuro-2 A cells, co-immunoprecipitation studies were unable to detect a stable association (data not shown).
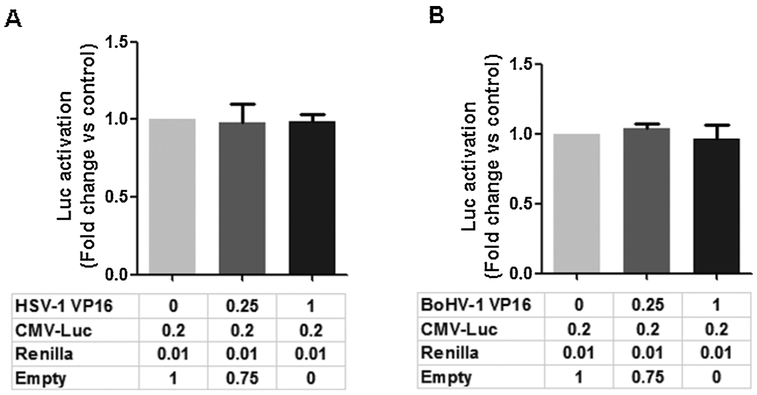
The ability of VP16 to regulate the human cytomegalovirus (CMV) immediate early promoter was examined in Neuro-2 A cells because this promoter regulated expression of S33Y and the wt β-catenin constructs used for this study. The pcDNA3-Flag-Luciferase construct used for this study contains the CMV promoter to drive expression of the luciferase gene and was used for this study. The HSV-1 (Fig. 6A) and BoHV-1 (Fig. 6B) had no effect on CMV promoter activity in Neuro-2 A cells. In summary, these studies demonstrated that VP16 stimulated β-catenin dependent transcription in HFL and Neuro-2 A cells when β-catenin was over-expressed.
Fig. 6. VP16 does not trans-activate the CMV IE promoter.
Neuro-2 A cells were cotransfected with 0.2 μg of the CMV- luciferase reporter and 0.01 μg of Renilla, and HSV-1VP16 (Panel A) or BoHV-1 VP16 (Panel B) at the designated amount. The CMV- luciferase construct (pcDNA3-FLAG-Luciferase) and Renilla luciferase under control of a minimal herpesvirus TK promoter (0.01 μg DNA) were normalized to a value of 1, and fold activation was calculated for the other samples. These results are the average of three independent experiments, and error bars denote standard errors.
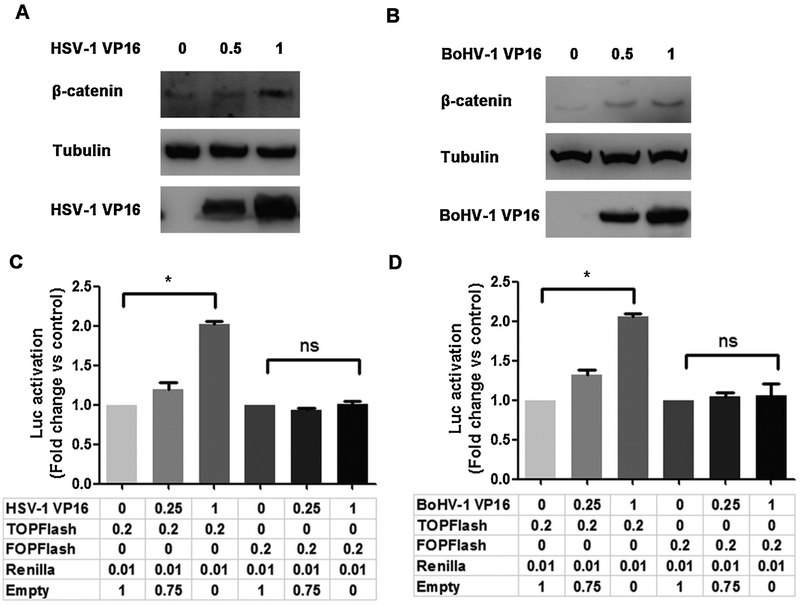
Additional studies tested whether VP16 increased endogenous β-catenin protein levels and β-catenin dependent transcription in transfected Neuro-2 A cells. VP16 encoded by HSV-1 (Fig. 7A) and BoHV-1 (Fig. 7B) increased steady levels of the β-catenin protein in transfected Neuro-2 A cells. HSV-1 (Fig. 6C) and BoHV-1 (Fig. 6D) encoded VP16 consistently increased β-catenin dependent transcription, as measured by TOPflash promoter activity. In contrast to TOPflash dependent transcription, VP16 (HSV-1 and BoHV-1) had no effect on FOPflash promoter activity indicating the effect was not merely due to VP16 increasing basal promoter activity. Relative to over-expression studies using the S33Y protein, the effects of VP16 on endogenous β-catenin protein levels and TOPflash promoter activity were modest further demonstrating endogenous levels of the β-catenin protein are unstable. Thus, VP16 in the absence of other viral genes stimulated β-catenin dependent transcription, in part by stabilizing β-catenin steady state protein levels.
Fig. 7. VP16 stimulates endogenous β-catenin signaling.
Neuro-2 A cells in 60mm dishes were transfected with VP16 encoded by HSV-1 (Panel A) or BoHV-1 (Panel B) at the designated concentrations (ug DNA). VP16 and β-catenin protein levels in whole cell lysate were examined at 48 h after transfection using antibodies described in the materials. VP16 protein levels were detected using the corresponding antibodies.
Neuro-2 A cells were cotransfected with 0.2 μg of the TOPFlash or FOPFlash luciferase reporter construct, 0.01 μg of Renilla reporter construct and HSV-1 VP16 (Panel C) or BoHV-1 VP16 (Panel D) at the designated concentrations. Results for cells transfected with 0.2 μg of the TOPFlash/FOPFlash luciferase reporter and Renilla luciferase under the control of a minimal herpesvirus TK promoter (0.01 μg DNA) were normalized to a value of 1, and fold activation was calculated for the samples. These results are an average of three independent experiments, and error bars denote standard errors. An * denotes significant differences (P < 0.05).
4. Discussion
In this study, we provided evidence that the β-catenin specific inhibitor (iCRT14) reduced HSV-1 replication in primary HLF and Vero cells suggesting an active Wnt/β-catenin signaling pathway enhances productive infection. Similar decreases in virus titers were detected when iCRT14 was added at the time of infection or 8 h after HLF cells were infected. These results implied that the effects of β-catenin on productive infection occurred at late times after infection, which is supported by increased β-catenin dependent transcription at late times after infection. In contrast, regulation of β-catenin protein levels was not the same in HFL versus Vero cells. For example, induction of β-catenin protein levels was higher in Vero cells at late times after infection relative to HFL cells. Furthermore, we observed an increase in β-catenin protein levels in HFL cells when incubated with UV-inactivated virus, which was unexpected. We suggest that when UV-inactivated virus interacted with HFL cells there was an increase in β-catenin protein levels in HFL cells because Wnt receptors are cell surface molecules. However, this was not activated β-catenin because we were unable to detect increased β-catenin dependent transcription in HFL cells after incubating with UV-inactivated virus. Since the β-catenin destruction complex is comprised of many proteins (Clevers and Nusse, 2012; Nusse and Clever, 2017), we attribute the differences in β-catenin protein levels observed in HFL versus Vero cells to cell type specific factors. This finding further underscores the importance of measuring β-catenin dependent transcription and not merely relying on making conclusions about increased β-catenin protein or RNA levels. In conclusion, these studies demonstrated that β-catenin dependent transcription was increased at late times after infection, which correlated with enhancing virus production.
In general, the Wnt/β-catenin signaling pathway promotes cell survival and cell growth (Ali-Harthi, 2012; Langhammer et al., 2013; Polakis et al., 2012), in part by stimulating Akt signaling pathways (Fukumoto et al., 2001; Kaler et al., 2009; Kim et al., 2007; Langhammer et al., 2013). It is also well established that an active Akt pathway promotes cell survival (Manning and Toker, 2017; Martelli et al., 2012). Based on these observations, we suggest that activating β-catenin at late times after infection enhances virus production by keeping infected cells alive longer. However, we cannot rule out the possibility that an active Wnt/β-catenin pathway also facilitates egress, virus assembly, or stimulates certain late viral promoters.
Although VP16 stimulates IE promoter activity by interacting with HCF-1 and Oct-1 (Kristie, 2007), VP16 has additional functions. For example, interactions between CREB binding protein and interferon regulatory factor 3 are disrupted by VP16 (Xing et al., 2013). Furthermore, VP16 regulates histone occupancy and histone acetylation on the HSV-1 genome (Hancock et al., 2010; Kutluay and Treizenberg, 2009) and stimulates chromatin remodeling and unfolding (Tumbar et al., 1999), in part by interacting with chromatin-modifying coactivators (Herrera and Treizenberg, 2004), including the SAGA histone acetylase complex (Hall and Struhl, 2002). VP16 also interacts with RNA PolII associated proteins, including Mediator (Mittler et al., 2003), TBP, and TFIIB (Hall and Struhl, 2002a). These interactions may indirectly stimulate β-catenin dependent transcription by making chromatin more accessible to β-catenin or certain coactivators. VP16 increased β-catenin steady state levels in productively infected Vero cells, transfected Neuro-2 A cells; however, to a lesser extent in HLF cells suggesting VP16 transcriptionally activates a β-catenin agonist or inhibits expression of β-catenin antagonists. Hence, the mechanism by which VP16 regulates the Wnt/pathway may be complicated.
Our previous studies concluded that the Wnt/β-catenin signaling pathway promotes certain aspects of the BoHV-1 latency-reactivation cycle (Liu et al., 2016), which appears counterintuitive to the finding that the same signaling pathway stimulates productive infection. However, the Wnt/β-catenin signaling pathway has tissue and cell-type specific functions that may enhance latency in neurons but stimulate productive infection in non-neuronal cell types. For example the Wnt/β-catenin signaling pathway promotes differentiation of neurons by stimulating axon growth and targeting axons to specific sites (Bamji et al., 2006; Bhardwaji et al., 2013; Murase et al., 2002; Purro et al., 2014; Salinas, 2012). Furthermore, the Wnt/β-catenin signaling pathway inhibits neuro-degeneration (Biechele et al., 2012; Chen et al., 2001; Nestor et al., 2005; Zhao et al., 2012). Conversely, the β-catenin signaling pathway stimulates cell growth, inhibits cell death, and promotes tumor growth when certain Wnt regulators are mutated (Clevers and Nusse, 2012; Hayward et al., 2008; Polakis et al., 2012). In conclusion, we suggest that cell type specific functions of the Wnt/β-catenin signaling pathway can mediate crucial events during the latency-reactivation cycle as well as productive infection.
Acknowledgements
This research was supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R21NS102290, USDA-NIFA Competitive Grants Program (13-01041 and 16-09370), the Sitlington Endowment, and support from the Oklahoma Center for Respiratory and Infectious Diseases (National Institutes of Health Centers for Biomedical Research Excellence Grant # P20GM103648). L.Z. was partially supported by Chinese National Science Foundation Grant (No. 31472172 and No. 31772743) and National Key Research Program (No. 2016YFD0500704).
References
- Agelidis A, Hadigal SR, Jaishankar D, Shukla D, 2015. Viral activation of heparanase drives pathogenesis of herpes simplex virus-1. Cell Rep. 20, 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-Harthi L, 2012. Wnt/beta-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuro-sychiatric disorders. J. Neuroimmine Pharm. 7, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Guerra MC, Ronchini C, Capobianco AJ, 2007. Mastermind like 1 is a specific coactivator of beta-catenin transcription activation and is essential for colon carcinoma cell survival. Cancer Res. 67, 8690–8698. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF, 2006. BDNF mobilizes synaptic vesicles and enhances synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J. Cell Biol. 174, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaji D, Nager M, Camats J, David M, Benguira A, dopazo A, Canti C, Herreros J, 2013. Chemokines induce axon outgrowth downstream of hepatocyte growth factor and TCF/beta-catenin signaling. Front. Cell. Neurosci. 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele TL, Kulikauskas RM, Toroni RA, Lucero OM, Swift RD, James RG, Robin NC, Dawson DW, Moon RT, Chien AJ, 2012. Wnt/beta-catenin signaling and AXIN1 regulate apoptosis mediated by inhibition of BRAFV600E kinase in human melanoma. Sci. Signal. 5 (206), ra203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush BM, Brock AT, Deng JA, Nelson RA Jr, Sumter TF, 2013. The Wnt/b-catenin/TCF pathway upregulates HMGA1 expression in colon cancer. Cell Biochem. Funct. 3, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang C-Y, 2001. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 152 97–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E-J, Kim S, Jho E, Song K-J, Kee S-H, 2013. Axin expression enhances herpes simplex virus type 1 replication by inhibiting virus-mediated cell death in L929 cells. J. Gen. Virol. 94, 1636–1646. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R, 2012. Wnt/B-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh C-M, Maemura K, Layne MD, Yet S-F, Lee K-H, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee M-E, 2001. Akt participation in the Wnt signaling pathway through dishevelled. J. Biol. Chem. 276, 17479–17483. [DOI] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BC, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AMC, DasGupta R, 2011. An RNAi-based chemical genetic screen identifies threee small-molecular inhibitors of the Wnt/wingless signalling pathway. Proc. Natl. Acad. Sci. U. S. A. 108, 5954–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Struhl K, 2002. The VP16 activation domain interacts with multiple transcriptional components as determined by protein-protein cross-linking in vivo. J. Biol. Chem. 277, 46043–46050. [DOI] [PubMed] [Google Scholar]
- Hancock M, Cliffe AR, Knipe DM, Smiley JR, 2010. Herpes simplex virus VP16, but not ICP0, is required to reduce histone occupancy and enhance histone acetylation on viral genomes in U2OS osteosarcoma cells. J. Virol. 84, 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P, Kalmar T, Arlas AM, 2008. Wnt/Notch signalling and information processing during development. Development 135, 411–424. [DOI] [PubMed] [Google Scholar]
- Herrera F, Treizenberg SJ, 2004. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J. Virol. 78, 9689–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler P, Godasi BN, Augenlicht L, Klampfer L, 2009. The NF-kB/Akt-dependent induction of Wnt signaling in colon cancer cells by macrophages and IL-1b. Cancer Microenviron. 2, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, He R, Venkatadri R, Forman M, Arav-Boger R, 2013. Wnt modulating agents inhibit human cytomegalovirus replication. Antimicrob. Agents Chemother. 57, 2761–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanon Y, Kypta R, 2003. Secreted antagonists of the Wnt signaling pathway. J. Cell Sci. 116 2677–2634. [DOI] [PubMed] [Google Scholar]
- Kim S-E, Lee W-J, Choi K-Y, 2007. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell. Signal. 19, 511–518. [DOI] [PubMed] [Google Scholar]
- Kristie TM, 2007. Early pre-initiation of alphaherpesvirus viral gene expression In: Campadelli-Fiume G, Mocarski E, Moore PS, Whitley R, Yamanishi K (Eds.), Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press, Cambridge, NY, pp. 112–127. [PubMed] [Google Scholar]
- Kristie TM, Roizman B, 1988. Differentiation and DNA contact points of host proteins binding at the cis site for virion-mediated induction of alpha genes of herpes simplex virus 1. J. Virol. 62, 1145–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie TM, Pomerantz JL, Twomey TC, Parent SA, Sharp PA, 1995. The cellular C1 factor of the herpes simplex virus enhancer complex is a family of polypeptides. J. Biol. Chem. 270, 4387–4394. [DOI] [PubMed] [Google Scholar]
- Kutluay S, Treizenberg SJ, 2009. Regulation of histone deposition on the herpes simplex virus type 1 genome during lytic infection. J. Virol. 83, 5835–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhammer T-S, Roolf C, Krohn S, Kretzchmar C, Huebner R, A, Rolfs, Freund, M., Junghass, C., 2013. PI3/K/Akt signalling interacts with Wnt/Beta-catenin signaling but does not induce an accumulation of beta-Catenin in the nucleus of acute lymphoblastic leukemia cell lines. Blood 122, 4886. [Google Scholar]
- Lemaster S, Roizman B, 1979. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J. Virol. 35, 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hancock M, Workman A, Doster A, Jones C, 2016. Beta-catenin, a transcription factor activated by canonical Wnt signaling, is expressed in sensory neurons of calves latently infected with bovine herpesvirus 1. J. Virol. 90, 3148–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B, Toker A, 2017. AKT/PKB signaling: navigating the network. Cell 169, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H, Stow ND, Preseton VG, Timbury MC, Wilkie NM, 1987. Physical mapping of herpes simplex virus-induced polypeptides. J. Virol. 28, 624–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A, Tabellini G, Bressanin D, Ognibene A, Goto K, Coco L, Evangelisti C, 2012. The emerging multiple roles of nuclear Akt. Biochemica et Biophysica Acta 1823, 2168–2178. [DOI] [PubMed] [Google Scholar]
- McLean G, Nixon F, Langeland N, Haarr L, Marsden H, 1990. Identification and characterization of the virion protein products of herpes simplex virus type 1 gene UL47. J. Gen. Virol. 71, 2953–2960. [DOI] [PubMed] [Google Scholar]
- Mittler G, Stuhler T, Santolin L, Uhlmann T, Krammer E, Lottspeich F, Berti L, Meisterernst M, 2003. A novel docking site on mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22, 6494–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM, 2002. Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35, 91–105. [DOI] [PubMed] [Google Scholar]
- Nestor T, Masckauchan H, Shawber CJ, Funahashi Y, Li C-M, Kitajewski J, 2005. Wnt/beta-catenin signaling induces proliferation, survival, and Interleukin-8 in human endothelial cells. Angiogenesis 8, 43–51. [DOI] [PubMed] [Google Scholar]
- Nusse R, Clever H, 2017. Wnt/beta-catenin signaling, diseases, and emerging therapeutic modalities. Cell 169, 985–999. [DOI] [PubMed] [Google Scholar]
- O’Hare P, Goding CR, 1988. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell 52, 435–445. [DOI] [PubMed] [Google Scholar]
- O’Hare P, Hayward GS, 1985. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J. Virol. 56, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan-Langston D, 2000. Herpes simplex of the ocular anterior segment. Curr. Clin. Top. Infect. Dis. 20, 298–324. [PubMed] [Google Scholar]
- Polakis P, 2012. Wnt signaling in cancer In: Nusse R, He X, van Amerongen R (Eds.), Cold Spring Harb Perspect Biol. Cold Spring Harbor Laboratory Press, pp. 1–13. [Google Scholar]
- Purro SA, Galli S, Salinas PC, 2014. Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. J. Mol. Cell Biol. 6, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, 2012. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perpect. Biol. 4, a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, McDonald HR, Nesburn AB, Minckler DS, 1980. Penetrating keratoplasty: changing indications, 1947 to 1978. Arch. Ophthalmol. 98, 1226–1229. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M, 2010. Differentiation of mouse Neuro-2A cells into dopamine neurons. J. Neurosci. Methods 186, 60–67. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Sudlow G, Belmont AS, 1999. Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J. Cell Biol. 145, 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT, 2003. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685. [DOI] [PubMed] [Google Scholar]
- Workman A, Zhu L, Keel BN, Smith TPL, Jones C, 2018. The Wnt signaling pathway is differentially expressed during the bovine herpesvirus 1 latency-reactivation cycle: evidence that two proteinkinases associated with neuronal survival, Akt3 and BMPR2, are expressed at higher levels during latency. J. Virol. 92, e01937–01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ni L, Wang S, Wang K, Lin R, Feng C, 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-kB activation and blocking IFN regualtory factor 3 to recruit is coactivator CBP. J. Virol. 87, 9788–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y, 2012. Activation of Akt/GSK-3beta/beta-catenin signaling pathway is invovlved in survival of neurons after traumatic brain injury in rats. Neurol. Res. 34, 400–407. [DOI] [PubMed] [Google Scholar]
- Zhu L, Jones C, 2017b. Canonical Wnt/beta-catenin signaling stimulates bovine herpesvirus 1 productive infection. Virology 500, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Workman A, Jones C, 2017a. A potential role for a beta-catenin coactivator (high mobility group AT-hook 1 protein) during the latency-reactivation cycle of bovine herpesvirus 1. J. Virol. 91, e02132–20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, 2001. Wnt signaling: antagonistic Dicckopf. Curr. Biol. 11, R592–R595. [DOI] [PubMed] [Google Scholar]