Figure 4. Pathological changes to the glymphatic pathway.
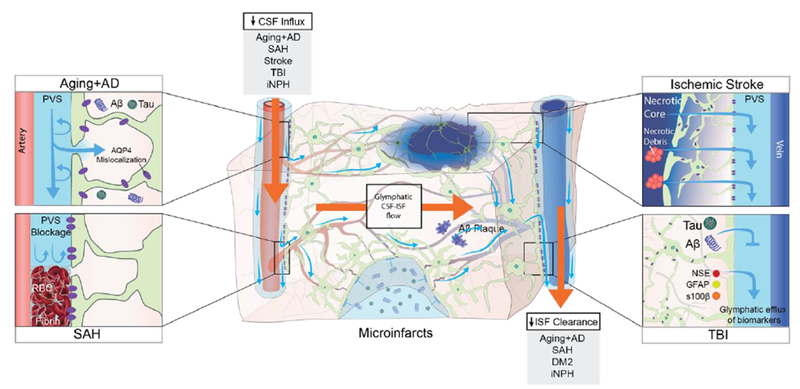
Aging and several diseases have been associated with a decrease in CSF influx to the glymphatic pathway and/or reduced clearance efficacy both in animals and in humans. In aging mice, the flow changes are likely caused by reduced vascular compliance, increased AQP4 expression and AQP4 mislocalization away from the astrocytic end-feet, which all cause reduced parenchymal influx of CSF.23 In a human postmortem study, AQP4 expression increased with age, albeit without AQP4 mislocalization.55 In murine models of AD, soluble and insoluble Aβ plaques provoke AQP4 mislocalization and impaired CSF influx.34,56 In AD patients, CSF clearance rate is reduced and exhibits an inverse relationship with Aβ levels.16 Post-mortem studies of AD patients identified AQP4 mislocalization and an increase in total AQP4 expression in AD patients compared to non-AD subjects.55 In hemorrhagic stroke in mice and gyrenchephalic non-human primates, blood components leaking into the PVS, especially fibrin/fibrinogen deposits, occlude the PVS, which leads to reduced CSF influx.61–63 In rodent models of ischemic stroke, necrotic cores are formed within the brain parenchyma, around which reactive astrocytes create a barrier (glial scar) to contain the injury and the toxic agents that form upon necrosis.64 Contents of the necrotic core leak through the permeable glial scar into the PVS.64 In mice, cerebral microinfarcts lead to a transient global reduction in glymphatic influx, and prolonged trapping of solutes within the infarct cores, probably due to reduced interstitial fluid turnover.60 TBI in mice leads to reduced glymphatic clearance, and biomarkers of the injured parenchyma are transported through the glymphatic pathway towards the cervical lymphatic system.42 In iNPH patients, glymphatic function is broadly impaired and characterized by both a delayed influx and a reduced clearance rate following intrathecal contrast injection.15 In rat models of diabetes mellitus type 2 (DM2), CSF tracers remain trapped within the brain parenchyma for prolonged periods, suggesting that perivenous efflux is decreased.68 This finding has not yet been replicated in humans, but we speculate that reduced brain clearance could contribute to the cognitive decline that is often seen in DM2 patients.
CSF, cerebrospinal fluid; ISF interstitial fluid, PVS: perivascular space; AQP4, aquaporin-4, AD, Alzheimer’s Disease; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; iNPH, idiopathic normalpressure hydrocephalus; DM2, diabetes mellitus type 2; GFAP, glial fibrillary acidic protein; S100B, S100 calcium binding protein; NSE, neuron-specific enolase.
