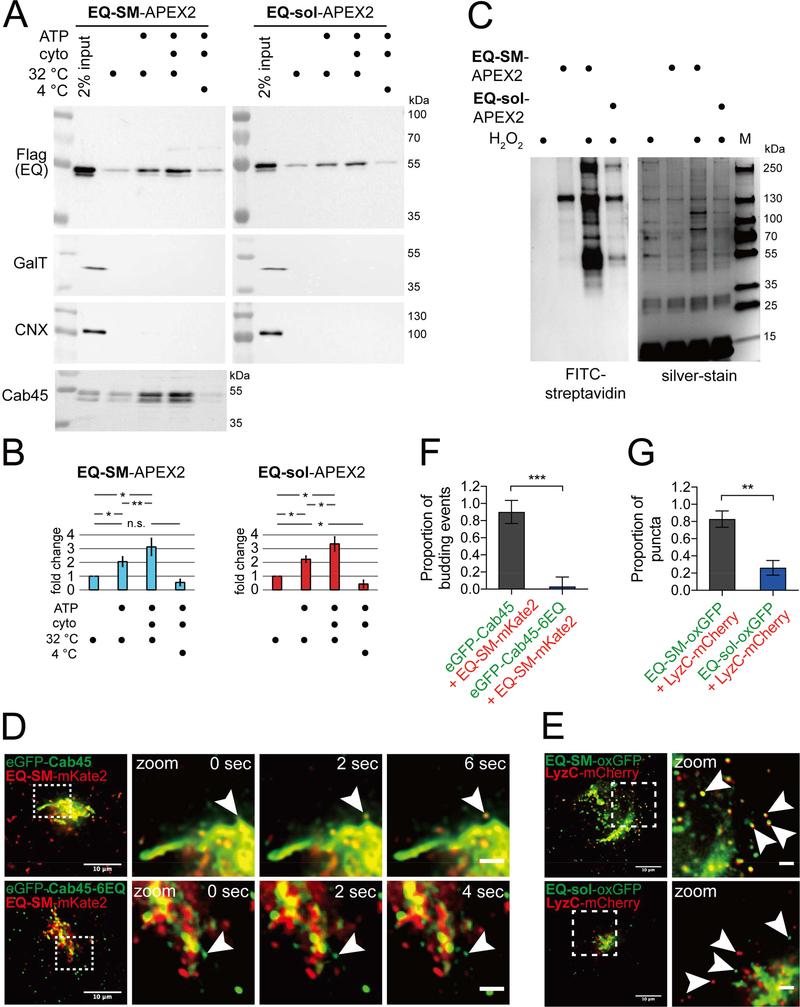
Figure 1. Comparative proteomics of Golgi-derived vesicles identifies Cab45 as a cargo of the sphingomyelin secretion pathway.
(A) Preparation of Golgi-derived vesicles. Cell lines expressing EQ-SM-APEX2 or EQ-sol-APEX2 were permeabilized and then incubated with rat liver cytosol, ATP generating reaction components, at 32ºC or on ice for 45 minutes, as indicated. Released vesicular material was collected by centrifugation and the relative proportions of EQ-SM-APEX2 or EQ-sol-APEX2, β1,4-galactosyltransferase (GalT; a resident of late Golgi compartments), calnexin (CNX; an ER resident), and Cab45, in each fraction were determined by immunoblotting. Note that EQ-SM-APEX2, EQ-sol-APEX2, and Cab45 are detected in the budded vesicle fractions, but GalT (a Golgi resident) and CNX (an ER resident) are not. An example experiment for EQ-SM-APEX2 and EQ-sol-APEX2 is shown. Proteomic analyses were performed for two independent vesicle preparations. See also Table S1. (B) Proportions of EQ-SM-APEX2 and EQ-sol-APEX2 in vesicle fractions. The fold increases in the amounts of EQ-SM-APEX2 and EQ-sol-APEX2 in the vesicle fractions (mean ± s.d.; 3 independent experiments) are plotted. (C) Purification of biotinylated proteins from vesicle fractions. Vesicle fractions from EQ-SM-APEX2 or EQ-sol-APEX2 expressing or control (no EQ probe) cells were incubated with APEX-mediated biotinylation reagents, unless otherwise noted. Detergent (1% TritonX-100 and 0.1% SDS) was added to solubilize vesicle-associated proteins and biotinylated proteins were purified using immobilized streptavidin. An aliquot of each purified fraction was separated by SDS-PAGE and proteins were visualized by silver stain. Biotinylated proteins in each fraction were identified by blotting with fluorescent streptavidin. The migration of protein molecular mass standards is indicated to the right of each gel. (D) Example micrographs of cells expressing eGFP-Cab45 and EQ-SM-mKate2. Each micrograph shows the Golgi and surrounding cytoplasm of cells expressing the indicated fluorescent proteins. Cab45–6EQ is an engineered variant of Cab45 that does not bind Ca2+. To quantify the proportion of Golgi-derived vesicles containing both proteins, time-lapse movies were acquired and examined to identify vesicular/tubular profiles that underwent budding and fission from the Golgi apparatus. The arrowheads indicate a budding vesicle. Data was collected from 3 independent experiments. Merge bars, 10 μm. Zoom bars, 2 μm. (E) LyzC is exported from the Golgi in vesicles marked by EQ-SM, but not EQ-sol. Images of the Golgi region of cells expressing mCherry-tagged LyzC and EQ-SM-oxGFP or EQ-sol-oxGFP are shown. Arrowheads point to post-Golgi cytoplasmic vesicles. Merge bars, 10 μm. Zoom bars, 2 μm. (F) The mean (± s.d.) proportion of 26 budding events from 4 cells expressing eGFP-Cab45 and EQ-SM as well as 32 budding events from 9 cells expressing eGFP-Cab45–6EQ and EQ-SM are plotted. Data was collected from 2 independent experiments. (G) The mean (± s.d.) proportion of detected postGolgi LyzC vesicles that were either EQ-SM (n=185 vesicles from 6 cells) or EQ-sol (n=146 vesicles from 5 cells) positive was quantified. Data was collected from 2 independent experiments.