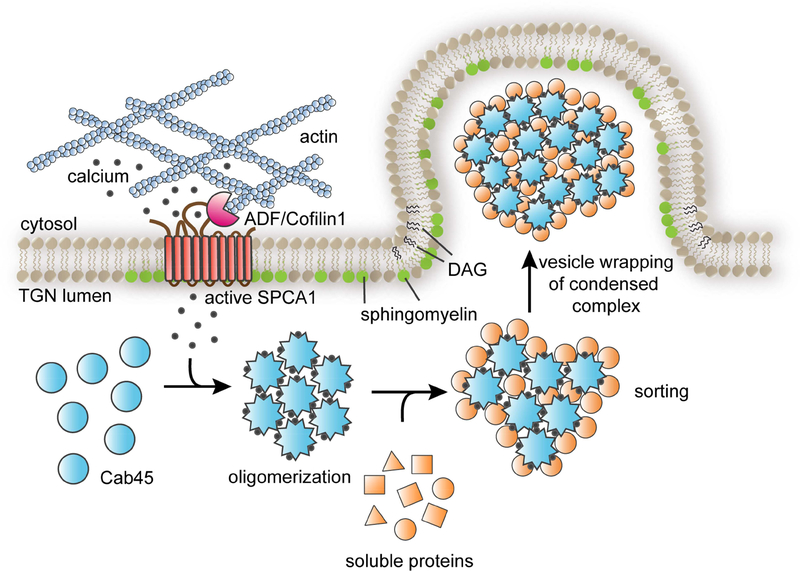
Figure 7. SPCA1 links sphingomyelin synthesis to Ca2+ dependent secretory protein and lipid sorting in the TGN.
The model depicts the events leading to the sorting and export of secretory cargo from the TGN, where the convergence of sphingomyelin synthesis and Ca2+ import into the lumen of the TGN drives the formation of a secretory carrier enriched in Cab45client complexes. Secretory cargo sorting is initiated by SPCA1-mediated Ca2+ influx, which is triggered by binding of ADF/cofilin1 to SPCA1 in the cytoplasm, where F-actin is associated with the TGN membrane. Synthesis of sphingomyelin in the TGN membrane potentiates SPCA1 mediated Ca2+ pumping in a region of the TGN membrane enriched in sphingomyelin. The local elevation of lumenal Ca2+ drives oligomerization of Cab45, which binds to soluble secretory protein clients, condensing them from the bulk milieu. The second product of SM synthesis, diacylglycerol (DAG), promotes engulfment of Cab45-client complexes by generating negative membrane curvature, leading to the formation of a secretory carrier enriched in oligomeric Cab45-client complexes.