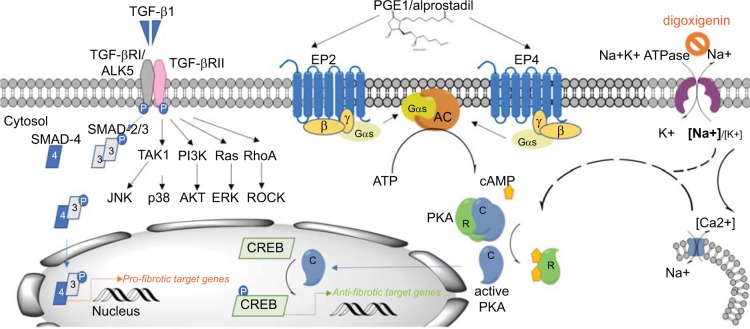
Fig 8. Summary binding of active TGF–β dimer to TGF–β receptor type II leads to the formation of a stable complex with the type I/ activin receptor–like kinase (ALK5) receptor and its transphosphorylation and activation.
In the case of the canonical TGF–β signaling pathway, this results in the activation of SMAD family transcription factors SMAD–2 and –3 which, upon phosphorylation, form complexes with SMAD–4 and translocate to the nucleus, where they act in transcriptional complexes as regulators of pro–fibrotic down–stream genes [6]. In our assay, TGF–β1 signaling involved ALK5 and SMAD3, but also all three mitogen–activated protein kinase (MAPK) pathways: e,g, the extracellular signal–regulated kinase (ERK), p38 MAPK, and c–Jun–N–terminal kinase (JNK) pathways. In addition, TGF–β1 effect was mediated by PI3 kinase / Akt and Rho GTPase pathways. Binding of alprostadil to the G–protein coupled receptors EP2 and EP4, which are linked to G–αs proteins, induced the adenylate cyclase–mediated cAMP second messenger formation from ATP. It is well established that cAMP acts by activating protein kinase A (PKA) [28, 29], resulting in dissociation of the regulatory (R) and the catalytic (C) subunits of the kinase. The catalytic subunits translocate to the nucleus and initiate the activation of the cAMP response element binding (CREB) transcription factor, and, as a consequence, transcription of downstream genes. Cardiac glycosides concentration–dependently increased the intracellular [Na+]i/[K+]i ratio, which led to an increase in COX2 expression and PKA activation and decreased α–SMA, COL1 and FN [30]. To which extent this effect was mediated by an increase in intracellular Ca2+ concentration through activation of the reverse mode of an Na+/Ca2+ exchanger warrants further clarification.