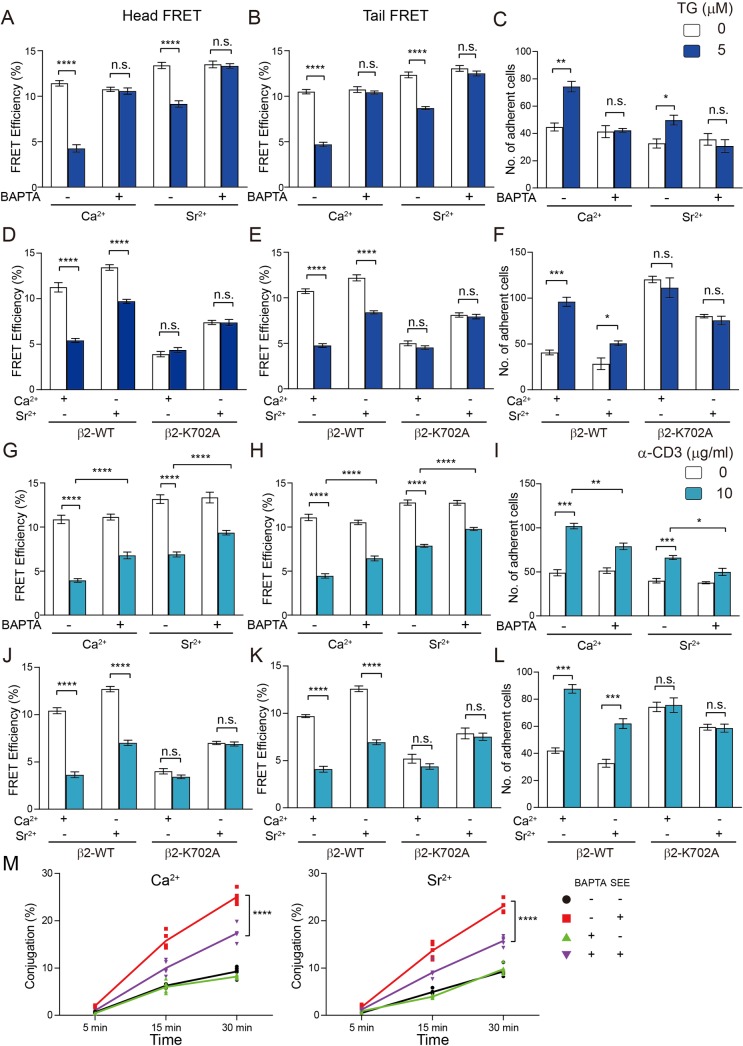
Fig 7. Intracellular Ca2+ activates αLβ2 by its charge.
TG (5 μM TG in A–F) or TCR stimulation (10 μg/ml α-CD3ε in G–L) was used to induce Ca2+ or Sr2+ influx in Jurkat T cells. Pretreatment of 20 μM BAPTA-AM (A–C, G–I, M) was used to chelate intracellular Ca2+ or Sr2+. The stimulating buffer contained Ca2+ at physiological concentration (1 mM) or Sr2+ (5 mM). We used a higher Sr2+ concentration because Sr2+ influx is less efficient than Ca2+ influx. β2-KO Jurkat cells were reconstituted with β2-WT or K702A mutant (D–F, J–L). (A, B, D, E) WT or K702A mutant αLβ2 conformational changes induced by TG stimulation were measured by the Head and Tail FRET assays. (C, F) The adhesion of Jurkat T cells to ICAM-1-coated surface induced by TG stimulation was measured by the flow chamber assay. (G, H, J, K) WT or K702A mutant αLβ2 conformational changes induced by TCR stimulation were measured by the Head and Tail FRET assays. (I, L) The adhesion of Jurkat T cells to ICAM-1 coated surface induced by TCR stimulation was measured by the flow chamber assay. (M) Conjugation between T cells and SEE pulsed B cells measured by flow cytometry. Jurkat T cells and Raji B cells were labeled with Cell Tracker Deep Red and Cell Tracker CSFE, respectively. Raji B cells were pretreated with 5 μg/ml SEE in FBS-free RMPI-1640 medium and then mixed with Jurkat T cells in HBSS containing either 1.26 mM CaCl2 or 5 mM SrCl2. Two-way ANOVA was used to compare the differences between the group with or without BAPTA treatment in different time points (n = 5 for each group). Data are representatives of three independent experiments and displayed as individual points. The underlying data of panel A–M can be found in http://dx.doi.org/10.17632/tg2622h9dd.1. (A–L) Data are representatives of two independent experiments and displayed as mean ± SEM. Student t test was used to analyze the differences between two groups. ****P < 0.0001, ***P < 0.001, **P < 0.01. BAPTA-AM, 1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis (acetoxymethyl ester); Ca2+, calcium ion; CaCl2, calcium chloride; CD, cytoplasmic domain; CFSE, 5-(and-6)-Carboxyfluorescein Diacetate, Succinimidyl Ester; FBS, fetal bovine serum; FRET, florescence resonance energy transfer; HBSS, Hank’s Balanced Salt Solution; ICAM-1, intercellular adhesion molecule 1; n.s., not significant; RMPI, Roswell Park Memorial Institute; SEE, Staphylococcus aureus Enterotoxin E; Sr2+, strontium ion; SrCl2, strontium chloride; TCR, T-cell receptor; TG, thapsigargin; WT, wild type