Fig. 2. Type I JAK2 inhibitor protects JAK2 from degradation and down-regulation.
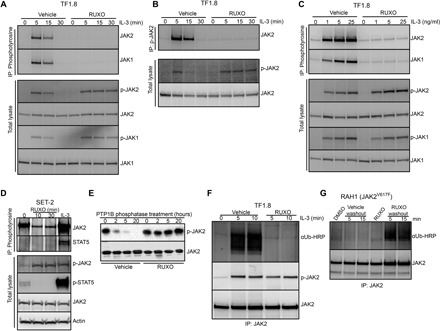
(A and B) TF1.8 cells were starved overnight in the presence of 0.5% FCS, preincubated with either vehicle or 280 nM ruxolitinib for 10 min, and stimulated with IL-3 (50 ng/ml) for different times. Cells were lysed and subjected to immunoprecipitation with (A) anti-phosphotyrosine (4G10) or (B) anti-phosphorylated JAK2 (Y1007/1008) antibodies, followed by immunoblotting with JAK2 and JAK1 antibodies. As a control, total lysates from the same experiment were immunoblotted with p-JAK1, p-JAK2, JAK1, and JAK2 antibodies as indicated. (C) TF1.8 cells were starved overnight in the presence of 0.5% FCS, preincubated with either vehicle or 280 nM ruxolitinib for 10 min, and stimulated with different doses of IL-3 for 5 min. Cells were lysed and subjected to immunoprecipitation (IP) with anti-phosphotyrosine (4G10) antibody, followed by immunoblotting with JAK1 and JAK2 antibodies. As a control, total lysates from the same experiment were immunoblotted with p-JAK1, p-JAK2, JAK1, and JAK2 antibodies. (D) SET-2 cells were incubated in 0.5% FCS for 6 hours before the addition of 280 nM ruxolitinib for 0, 10, or 30 min or stimulation with IL-3 (50 ng/ml) and EPO for 5 min. Cells were lysed and subjected to immunoprecipitation with anti-phosphotyrosine (4G10) antibody, followed by immunoblotting with JAK2 and STAT5 antibodies. As a control, total lysates from the same experiment were immunoblotted with p-JAK2, p-STAT5, JAK2, and actin antibodies. (E) Recombinant JAK2 kinase domain was mixed with recombinant tyrosine phosphatase PTP1B and ruxolitinib or no inhibitor in phosphatase assay buffer. Phosphatase reactions were incubated at room temperature for 0, 2, 5, and 20 hours, fractionated by SDS–polyacrylamide gel electrophoresis (PAGE), and immunoblotted with p-JAK2 and JAK2 antibody. (F) TF1.8 cells were starved overnight in the presence of 0.5% FCS, preincubated for 10 min with MG132 plus either vehicle or 280 nM ruxolitinib, and stimulated with IL-3 (50 ng/ml) for 0, 5, or 10 min. Cells were lysed and subjected to immunoprecipitation with JAK2 antibody, followed by immunoblotting with ubiquitin antibody conjugated to horseradish peroxidase (αUb-HRP) or p-JAK2 antibody. (G) Mononuclear cells from patient with myelofibrosis RAH1 were cultured in 10% FCS with EPO and IL-3 (1 ng/ml each) and 280 nM ruxolitinib or DMSO for 12 hours. Cells were then washed in cold RPMI and cultured in MG132 without additives for 5 or 15 min. Cells were lysed and subjected to immunoprecipitation with JAK2 antibody, followed by immunoblotting with ubiquitin-HRP or JAK2 antibody.
