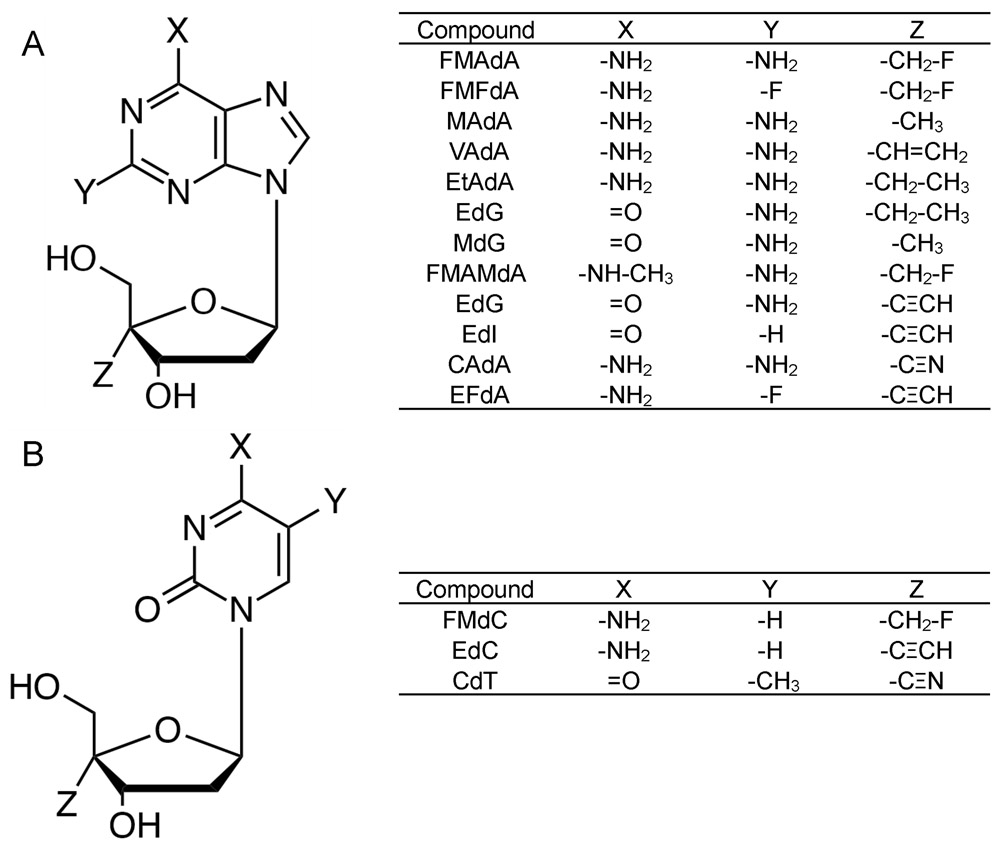
Figure 1.
Structures of 4′-NRTIs. All purine (A) and pyrimidine (B) derivatives possess a 4′-modified moiety. FMAdA; 4′-fluoromethyl-2-amino-2′-deoxyadenosine, FMFdA; 4′-fluoromethyl-2-fluoro-2′-deoxyadenosine, MAdA; 4′-methyl-2-amino-2′-deoxyadenosine, VAdA; 4′-vinyl-2-amino-2′-deoxyadenosine, EtAdA; 4′-ethyl-2-amino-2′-deoxyadenosine, EtdG; 4′-ethyl-2′-deoxyguanosine, MdG; 4′-methyl-2′-deoxyguanosine, FMAMdA; 4′-fluoromethyl-2-amino-N6-methyl-2′-deoxyadenosine. EdG; 4′-ethynyl-2′-deoxyguanosine, EdI; 4′-ethynyl-2′-deoxyinosine, CAdA; 4′-cyano-2-amino-2′-deoxyadenosine, EFdA; 4′-ethynyl-2-fluoro-2′-deoxyadenosine, FMdC; 4′-fluoromethyl-2′-deoxycytidine, EdC; 4′-ethynyl-2′-deoxycytidine, CdT; 4′-cyano-2′-deoxythymidine.