Abstract
Background:
Partner involvement can influence positive airway pressure (PAP) therapy use among patients with obstructive sleep apnea (OSA). This study assessed the feasibility, acceptability, and preliminary efficacy of a couples-oriented education and support (CES) intervention for PAP adherence.
Participants:
Thirty newly diagnosed OSA patients and their partners were randomly assigned to one of three groups: an education and support intervention directed at both patient and partner (CES), an education and support intervention directed only at the patient (PES), or usual care (UC).
Methods:
Feasibility and acceptability were assessed through enrollment and posttreatment program evaluations, respectively. Assessments of sleep quality, daytime sleepiness, and daytime function were obtained from both patients and partners at baseline and 3 months after PAP initiation. Objective PAP adherence was assessed at 1 week, 1 month, and 3 months.
Results:
Recruitment and attrition data suggest adequate feasibility. All patients and partners in the CES group reported that the intervention was helpful. Patients in the CES and PES groups increased their PAP adherence over the first month of treatment, whereas PAP adherence decreased over this period in the UC group. For patients, large to medium effects for sleep quality (d = −1.01), daytime sleepiness (d = −0.51), and daytime function (d = 0.51) were found for the CES group. The PES and UC groups effect sizes were large to small for sleep quality (d = −0.94; d = −0.40), daytime sleepiness (d = −0.42; d = −0.82), and daytime function (d = 0.41; d = 0.57), respectively. For partners, large effects for daytime sleepiness (d = −1.31) and daytime function (d = 1.54) and small to medium effect for sleep quality (d = −0.31) were found for the CES group. Worsening of sleep quality (d = 0.65) and no change in daytime sleepiness or daytime function were found for the PES group. For the UC group, medium to large effects were found for sleep quality (d = −0.77), daytime sleepiness (d = −0.77), and daytime function (d = 0.65).
Conclusions:
The findings of this pilot study provide support for taking a couples intervention approach to improve PAP adherence.
Positive airway pressure (PAP) therapy is the first-line treatment for obstructive sleep apnea (OSA) and has been shown to reduce respiratory disturbances and daytime sleepiness while improving quality of life, daytime function, and sleep quality (Antic et al., 2011; Giles et al., 2006). Despite its efficacy, initiation and continued use of PAP can be challenging for many patients; PAP is a demanding treatment. Up to 50% of OSA patients reject PAP or discontinue treatment within the first week and 29%–83% who continue treatment are nonadherent when adequate nightly use is defined as at least 4 hr per night (Engleman & Wild, 2003; Weaver & Grunstein, 2008; Wolkove, Baltzan, Kamel, Dabrusin, & Palayew, 2008). A dose-response relationship between PAP adherence and health outcomes including daytime sleepiness, memory, daytime function, and blood pressure has been demonstrated, with > 5 hr of nightly use associated with substantial improvements (Antic et al., 2011; Barbé et al., 2010; Weaver et al., 2007; Zimmerman, Arnedt, Stanchina, Millman, & Aloia, 2006). Given the importance of PAP adherence for health outcomes, promotion of PAP adherence using educational, technological, and behavioral intervention strategies comprises a growing body of literature and has shown some promise (Sawyer et al., 2011; Wozniak, Lasserson, & Smith, 2014); however, intervention strategies that target social factors that influence PAP adherence have received little attention (Crawford, Espie, Bartlett, & Grunstein, 2014).
For patients with chronic disease, spouses or partners are frequently described as the greatest source of social support for both the physical and emotional aspects of illness (Berg & Upchurch, 2007; Revenson, Kayser, & Bodenmann, 2005). Partners can influence patients’ health behaviors either positively or negatively depending on the type of involvement (encouraging and collaborating vs. criticizing and nagging; Franks et al., 2006; Helgeson, Novak, Lepore, & Eton, 2004; Khan, Stephens, Franks, Rook, & Salem, 2013; Lewis & Butterfield, 2007; Stephens et al., 2013). Consistent with those findings, available studies in OSA patients suggest that collaborative and supportive partner involvement can facilitate PAP use (Luyster, 2017). Inclusion of partners has been identified by OSA patients and their partners as a desired component for future PAP adherence interventions (Luyster et al., 2016). Partners have also reported improvements in their sleep and daytime functioning following patients’ PAP treatment initiation (Luyster, 2017). However, patients’ adherence to PAP is likely a mediating factor in this association; partners are likely to benefit from PAP treatment as a result of improvements in patients’ OSA symptoms, which will only occur with adequate PAP use. Thus, inclusion of partners in interventions aimed at improving PAP adherence may offer a novel approach to supporting the patient’s adoption and continued use of PAP and consequently lead to improvements in outcomes for both patients and partners.
Data from couples-oriented interventions in chronic diseases including cancer, arthritis, type 2 diabetes, cardiovascular disease, human immunodeficiency virus, and chronic pain have demonstrated small beneficial effects on patients’ psychological and physical symptoms, relationships, and improvements in adherence to diet and exercise recommendations and medications (Martire, Hemphill, & Polenick, 2016). Although the benefits to partners are less extensively examined, couples-oriented interventions have been shown to improve partners’ psychological functioning in terms of self-efficacy, stress, mastery, anxiety, and perceptions of marital quality and coping as a couple (Martire, Schulz, Helgeson, Small, & Saghafi, 2010).
The purpose of the current study was to assess the feasibility, acceptability, and preliminary efficacy of a couples-oriented education and support (CES) intervention for PAP adherence in a small randomized, controlled trial in couples, in which one partner was diagnosed with OSA and was beginning PAP therapy. More specifically, we describe the effects of a CES intervention relative to a patient-oriented education and support (PES) intervention that did not involve partners, and also relative to usual care (UC), in terms of effects on patients’ and partners’ sleep and daytime functioning and patients’ PAP adherence.
Methods
Participants
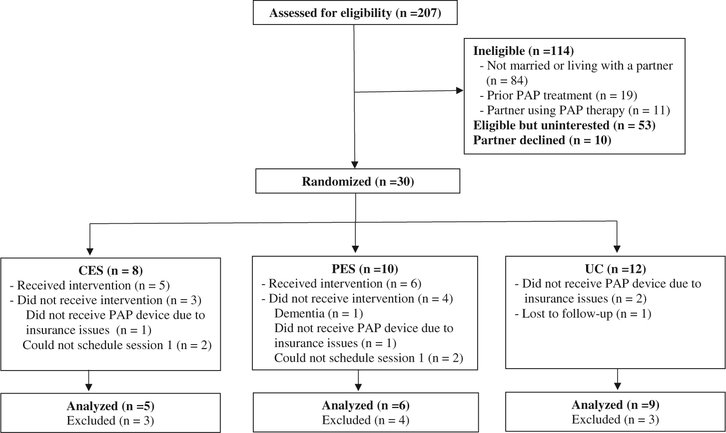
Patients were eligible if they (a) were 18 years of age or older, (b) diagnosed with OSA (apneahypopnea index (AHI) ≥ 5), (c) had a prescription for and acceptance (i.e., received a PAP device) of PAP therapy, (d) were married or living with a partner, and (e) were able to read and write English. Partners were eligible if they (a) were 18 years of age or older and (b) spoke, read, and wrote in English. Patients were excluded if they had prior treatment with PAP therapy and partners were excluded if they were currently being treated with PAP therapy (Figure 1).
Figure 1. Participant flow chart.
Procedures
The study protocol was approved by the Institutional Review Board at the University of Pittsburgh, and verbal informed consent was obtained from all participants. Patients were recruited from two academic sleep centers. Patients were initially approached in the sleep laboratories prior to their PAP titration night. Patients were given a brief overview of the study at that time and, if permission was granted, received a follow-up phone call in 2–3 days to determine interest in participating in the study. A telephone oral consent process was conducted first with patients and then with partners. Consenting patients and partners completed questionnaires separately at baseline and 3 months after PAP initiation and returned them in the mail. Couples were randomly assigned to one of three groups: CES, PES, or UC. Randomization was stratified by patient sex. Because this was a small pilot study, one respiratory therapist delivered both the CES and PES interventions. Training for intervention delivery was provided by the study PI (FSL). Delivery of the CES and PES interventions were guided by a protocol and script templates.
Interventions
Couples-oriented education and support
The CES protocol was informed by focus groups conducted in our prior work (Luyster et al., 2016), which suggested that inclusion of partners, education regarding sleep apnea, consequences of sleep apnea, benefits of PAP, and early feedback on patients’ PAP usage would be important components of a new PAP user program. Additionally, the CES protocol incorporated some components of motivational enhancement (ME) therapy (Aloia, Arnedt, Riggs, Hecht, & Borrelli, 2004), in particular information exchange, exploring potential barriers to PAP use, and goal setting, Utilizing the ME therapy format, the CES intervention consisted of two 1-hr face-to-face sessions and a 20-min follow-up telephone call with a trained respiratory therapist. The face-to-face sessions were attended by both patient and spouse or significant other. The telephone session was conducted individually on a 1-to-1 basis. The first face-to-face session occurred before the patient’s PAP setup. The second face-to-face session occurred 1 week after PAP setup. The telephone call occurred 1 week after the second face-to-face session. The aim of the first session was to provide patients and partners with knowledge, skills, and encouragement to begin PAP therapy. Major components of the session included (a) educational videos about OSA and PAP, (b) demonstration of PAP machine and equipment, (c) PAP concerns, and (d) goal setting. More specifically, patients and partners were given a list of common concerns about starting PAP treatment and were asked to individually identify their concerns about the patient starting PAP. Next, the couple was encouraged to collaboratively develop strategies for addressing each concern. For the goal-setting component, the patient set a goal for the first week of using PAP. Individually, patients and partners were asked to identify ways that the partner could help the patient achieve his or her goal or overcome potential barriers to achieving the goal. The couple was provided a list of potential ways the partner could help the patient (e.g., helping adjust mask and straps, giving verbal reminders to put mask on at bedtime, working with the partner to collaboratively resolve issues, encouraging use of PAP) but were encouraged to come up with other strategies that were not on the list. Similarities in strategies were highlighted and the couple was encouraged to discuss what strategies would work best for them. At the end of the first session, patients and partners were provided a sleep diary to monitor sleep–wake patterns during the first week of PAP use.
The aim of the second session was to provide information and encouragement to continue with PAP therapy. This session included (a) review of the patient’s diagnostic and titration night sleep study results highlighting changes in AHI, (b) review of PAP adherence report for the first week of therapy, (c) identification of barriers of PAP use, (d) highlighting changes in sleep quality or mood noted in patient’s and partner’s sleep diary, and (e) goal setting. More specifically, patients and partners individually identified barriers to the patient using PAP and then were encouraged to collaboratively identify strategies for supporting the patient’s routine use of PAP. The goal-setting activities were the same as in session 1, except the patient set a goal for the second week of PAP use.
The telephone session with the patient included (a) discussion of self-reported PAP use and (b) review of week 2 goal, while (c) encouraging use of previously identified strategies to overcome any barriers if patient did not met week 2 goal. The telephone session with the partner included (a) discussion of the patient’s use of PAP, (b) identification of things that have helped the patient use PAP since previous session, with reinforcement of any assistance provided by the partner, and (c) identification of barriers to patient’s use of PAP and discussion of strategies the partner could use to help the patient overcome these barriers.
Patient-oriented education and support
The patient-oriented education and support (PES) protocol covered all of the components of the CES protocol. Partners did not participate in the PES face-to-face sessions or the follow-up telephone call. Topics regarding identification of concerns about starting PAP, goal setting, barriers and facilitators of PAP use, and strategies for addressing concerns and barriers were patient-focused.
Usual care
Patients with OSA in the UC group received a routine follow-up sleep physician visit, and subsequent visits if necessary or requested by the patient. No alternations in the usual care provided by the physician were made. Couples in this group completed follow-up assessment.
Patient measures
PAP adherence
All patients were using a PAP device with remote monitoring capabilities. Objective adherence data were automatically uploaded to a secure data center daily. Adherence data were accessed through a web-based patient adherence management system at 1 week, 1 month, and 3 months. Adherence was defined as the average nightly use of PAP during each of the time intervals. We also divided patients into those with on average ≥ 4 hr of PAP use per night (good adherence) and those with < 4 hr of PAP use per night (poor adherence).
Measures for both patients and partners
Demographic and medical information were collected through self-report. AHI and BMI data were collected from patient medical records.
Feasibility and intervention acceptability
Feasibility was measured through rates of study enrollment and participation. Patients and partners in the CES group completed posttreatment ratings 2 weeks after PAP initiation in the following areas: (a) improvement in understanding of OSA and PAP therapy, patients’ concerns about starting PAP therapy, ability to provide assistance to patient, agreement to use PAP regularly, and agreement that attending sessions was beneficial (1 = strongly disagree to 5 = strongly agree), (b) helpfulness of session components and program to others with OSA (1 = not at all helpful to 5 = a great deal helpful), and (c) satisfaction with sessions led by the respiratory therapist (1 = not at all satisfied to 5 = a great deal satisfied).
Epworth Sleepiness Scale
Excessive daytime sleepiness was assessed with the 8-item Epworth Sleepiness Scale (ESS; Johns, 1991). The ESS evaluates the likelihood of dozing in eight different situations. Item responses are rated on a 0 to 3 scale with higher scores indicating more severe sleepiness. Total scores range from 0 to 24. A score of > 10 indicates excessive daytime sleepiness.
Pittsburgh Sleep Quality Index
Sleep quality was measured with the 18-item Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI assesses sleep quality during the past month and contains seven component scales: subjective sleep quality, sleep efficiency, sleep latency, sleep duration, sleep disturbances, daytime dysfunction, and use of sleep medications. Each component is scored from 0 to 3. A total global sleep quality score is calculated by summing the seven component scores, with scores ranging from 0 to 21. Higher scores indicate worse sleep quality. A global PSQI score greater than 5 differentiates good from poor sleepers (Buysse et al., 1989).
Functional Outcomes of Sleep Questionnaire
Daily functioning was assessed by the Functional Outcomes of Sleep Questionnaire (FOSQ-10; Chasens, Ratcliffe, & Weaver, 2009). The FOSQ-10 contains 10 items measuring the impact of excessive daytime sleepiness on daily tasks and roles in five domains: activity level, vigilance, intimacy and sexual relationships, general productivity, and social outcome. Each domain score ranges from 1 to 4 (1 indicating more difficulty) and the total score ranges from 5 to 20, with higher scores indicating greater functioning.
Statistical analyses
Descriptive statistics including counts and percentages and mean ± standard deviation (SD) were computed for recruitment rates and partner involvement and pre–post means on outcome and acceptability measures. Baseline comparisons examined differences between study completers and noncompleters and between the treatment groups (for patients and partners separately) using Fisher’s Exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Because of the small sample size, significance tests were not calculated for between- group comparisons of pre–post change in the primary analyses and only complete data (i.e., participants who completed baseline and follow-up assessments) were used. However, there was missing data for the following: 2 patients and 6 partners had missing data on the PSQI due to noncompletion of the instrument or missing data on individual items, which precluded calculation of the total score; 1 partner did not complete the ESS at follow-up; 1 patient and 1 partner did not complete the FOSQ at follow-up and baseline, respectively; for 1 patient, PAP adherence data could not be retrieved at 1 month; and for 1 patient PAP adherence could not be retrieved for any of the time points. Change scores (posttreatment–pretreatment) were calculated for each person. Within-group effect sizes were then calculated (mean change score–change score SD) using Hedge’s correction for small sample size (Hedges & Olkin, 1985). For the PSQI and ESS, a negative effect size indicates an improvement in symptoms and a positive effect size indicates a worsening of symptoms. For the FOSQ and PAP adherence variables, a positive effect size indicates an improvement in symptoms or PAP use and a negative effect size indicates a worsening of symptoms or PAP use. An effect size of 0.2 is considered small, 0.5 medium, and 0.8 large (Jenkinson, Davies, Mullins, & Stradling, 1999). SPSS 24 for Windows (IBM Corp., Armonk, NY) was used to conduct the statistical analyses.
Results
Baseline characteristics
Table 1 presents the baseline demographic and clinical information for patients. The majority of patients were male (66.7%), White (66.7%), married, highly educated, and employed. On average, patients were obese (BMI: 35.9 ± 8.4 kg/m2) and had severe OSA (AHI: 33.0 ± 27.3). Most partners were of the opposite sex (65% female) and similar to patients in age (M = 52.1 years, SD = 12.7) and race (57% White), but were less educated (48% > high school). Patients and partners in the CES, PES, and UC groups did not differ on demographic, medical, or baseline outcome variables (p values ≥ 0.05). Completers and noncompleters (patients and partners) did not differ significantly or clinically on demographic, medical, or baseline outcome variables for patients and partners (p values ≥ 0.05). Among study completers, 3 out of 5 partners in the CES group reported sharing the bed with their partner every night at baseline, which increased to 4 out of 5 partners at 3-month follow-up. There was no change from baseline to 3-month follow-up in the number of partners in the PES and UC groups who reported sharing the bed with their partner every night (PES: 4 out of 6; UC: 6 out of 9).
Table 1.
Baseline characteristics of patients.
| Usual Care (n = 12) |
Patient-oriented (n = 10) |
Couple-oriented (n = 8) |
Total (n = 30) |
|
|---|---|---|---|---|
| Age, mean (SD) | 54.0 (10.7) | 53.3 (12.1) | 53.1 (15.7) | 53.5 (12.2) |
| Male, n (%) | 8 (66.7%) | 6 (60.0%) | 6 (75.0%) | 20 (66.7%) |
| White, n (%) | 7 (58.3%) | 6 (60.0%) | 7 (87.5%) | 20 (66.7%) |
| Marital Status, n (%) | ||||
| Married | 8 (66.7%) | 6 (60.0%) | 7 (87.5%) | 21 (70.0%) |
| Living with partner | 4 (33.3%) | 4 (40.0%) | 1 (12.5%) | 9 (30.0%) |
| Employment Status, n (%) | ||||
| Full/part time | 8 (66.7%) | 5 (50.0%) | 6 (75.0%) | 19 (63.3%) |
| Unemployed | 2 (16.7%) | 2 (20.0%) | 1 (12.5%) | 5 (16.7%) |
| Retired | 2 (16.7%) | 3 (30.0%) | 1 (12.5%) | 6 (20.0%) |
| Education, n (%) | ||||
| High school or GED | 2 (16.7%) | 4 (40.0%) | 2 (25.0%) | 8 (26.7%) |
| College degree | 8 (66.7%) | 3 (30.0%) | 2 (25.0%) | 13 (43.3%) |
| Graduate degree | 2 (16.7%) | 2 (20.0%) | 5 (50.0%) | 9 (30.0%) |
| BMI, mean (5D) | 38.0 (6.6) | 34.8 (10.5) | 34.4 (8.3) | 35.9 (8.4) |
| AHI, mean (5D) | 38.5 (26.0) | 28.5 (25.9) | 30.4 (32.7) | 33.0 (27.3) |
| Diabetes, n (%)a | 3 (25.0%) | 2 (30.0%) | 1 (12.5%) | 7 (23.3%) |
| High blood pressure, n (%)a | 6 (50.0%) | 5 (50.0%) | 4 (50.0%) | 15 (57.7%) |
| High cholesterol, n (%)a | 7 (58.3%) | 2 (20.0%) | 4 (50.0%) | 13 (43.3%) |
n = 26.
AHI, apnea-hypopnea index; BMI, body mass index.
Note. No statistically significant differences between groups were found.
Feasibility and acceptability
Feasibility
Figure 1 shows study enrollment and follow-up. Of the 207 patients screened for eligibility, 114 (55%) were excluded. Of the remaining 93 eligible patients, 53 (57%) did not agree to participate in the study because of lack of interest, lack of time, or no reason indicated. Ten (11%) partners did not agree to participate in the study. A total of 30 couples (32.3%) consented to the study and were randomized, and a total of 20 couples completed the study (5 in the CES group; 6 in the PES group; 9 in the UC group). All 11 couples who started the CES and PES sessions completed all 3 sessions and follow-up questionnaires. Of the 9 couples who did not complete the study, 4 couples could not have their first intervention session scheduled before the patient received PAP, the patient would not be receiving PAP therapy due to insurance issues in 4 couples, and 1 patient developed dementia that precluded her from continuing in the study.
Acceptability
Table 2 presents the results from the satisfaction survey administered to patients and partners in the CES group. The majority of patients reported that the intervention improved their understanding of OSA and PAP and helped them feel more comfortable using PAP regularly. Most patients found discussion of PAP concerns and problems and viewing a demonstration of PAP equipment “much” or “very much” helpful. All partners reported that the intervention improved their understanding of OSA and PAP and their partner’s concerns about starting PAP and improved their ability to assist their partner. All partners found discussion of concerns about partner starting PAP and changes in their daytime functioning and sleep resulting from PAP, viewing demonstration of PAP equipment, and identification of ways to help partner meet weekly goals “very much” helpful.
Table 2.
Satisfaction survey completed by patients and partners in CES group.
| Patients (n = 5) | |
|---|---|
| % rating as ≥ “agree” (4; range = 1–5) | |
| Improved understanding of sleep apnea and its effects on health | 100% |
| Improved understanding of PAP and its effects on health | 100% |
| Helped feel more comfortable starting PAP treatment | 100% |
| Attending the sessions with partner was beneficial | 100% |
| Using PAP regularly has become less difficult | 80% |
| % reporting activity as ≥ “much helpful” (4; range = 1–5) | |
| Talking about concerns with starting PAP | 100% |
| Demonstration of PAP equipment | 100% |
| Setting weekly goals for the first and second weeks of PAP treatment | 80% |
| Talking about problems encountered while using PAP | 100% |
| Helpfulness of program for other people with sleep apnea | 100% |
| % rating as ≥ “much satisfied” (4; range = 1–5) | |
| Satisfaction with the sessions provided by the respiratory therapist | 100% |
| Partners (n = 5) | |
| % rating as ≥ “agree” (4; range = 1–5) | |
| Improved understanding of sleep apnea and its effects on health | 100% |
| Improved understanding of PAP and its effects on health | 100% |
| Improved understanding of partner’s concern about starting PAP | 100% |
| Improved ability to provide assistance to partner | 100% |
| Attending the sessions was beneficial | 100% |
| % reporting activity as ≥ “much helpful” (4; range = 1–5) | |
| Talking about concerns with partner starting PAP | 100% |
| Demonstration of PAP equipment | 100% |
| Identifying ways to help partner meet weekly goals | 100% |
| Talking about changes in mood, energy, daytime sleepiness, and sleep since partner started PAP | 100% |
| Helpfulness of program for partner of people with sleep apnea | 100% |
| % rating as ≥ “much satisfied” (4; range = 1–5) | |
| Satisfaction with the sessions provided by the respiratory therapist | 100% |
CES, Couple-oriented education and support intervention; PAP, positive airway pressure.
Patient outcomes
PAP adherence
Means, change scores, and within-group effect sizes for PAP adherence variables are displayed in Table 4. For the CES group, a medium increase in hours of PAP use of 1.4 hr from 1 week to 1 month was observed, but a medium to large decrease of 1.6 hr occurred from 1 month to 3 months. A similar pattern was observed for percentage of days with ≥ 4 hr. The PES group had a medium increase in hours of PAP use from 1 week to 1 month and a medium to large decrease from 1 month to 3 months. A similar pattern was observed for percentage of days with ≥ 4 hr, except for a small increase that was observed from 1 week to 1 month. In the UC group, a large decrease in hours of CPAP use was observed from 1 week to 1 month and a small decrease was observed 1 month to 3 months. A similar pattern was observed for percentage of days with ≥ 4 hr. When applying Medicare’s definition of adherence (≥ 4 hr of CPAP usage each night for ≥ 70% of nights) at 3-month follow-up, 37.5% met criteria in the CES group, 40% in the PES group, and 41.7% in the UC group.
Table 4.
PAP adherence at 1 week, 1 month, and 3 months.
| 1 week, Mean (SD) | 1 month, Mean (SD) | Change from 1 week to 1 month, Mean (95% Cl) | d | 3 months, Mean (SD) | Change from 1 month to 3 months, Mean (95% Cl) | d | |
|---|---|---|---|---|---|---|---|
| PAP use, hours | |||||||
| CES | 5.6 (2.8) | 6.1 (0.8) | 1.4 (0.2 to 2.5) | 0.52 | 5.3 (2.9) | −1.6 (−2.8 to −0.4) | −0.63 |
| PES | 4.5 (2.6) | 5.0 (2.9) | 0.5 (−0.5 to 1.5) | 0.50 | 4.5 (2.8) | −0.5 (−1.6 to 0.6) | −0.63 |
| UC | 5.8 (2.4) | 5.1 (2.3) | −0.7 (−1.5 to 0.1) | −0.92 | 4.8 (2.4) | −0.3 (−1.1 to 0.5) | −0.26 |
| Days with ≥ 4 hours, % | |||||||
| CES | 67.2 (35.8) | 75.0 (27.0)b | 16.0 (−1.9 to 34.0) | 0.38 | 71.9 (36.5) | −10.2 (−24.9 to 4.5) | −0.63 |
| PES | 66.0 (42.2)a | 71.2 (40.5)a | 5.2 (−10.8 to 21.3) | 0.25 | 62.3 (37.4)a | −8.9 (−22.1 to 4.2) | −0.63 |
| UC | 77.2 (35.0) | 66.8 (32.4) | −10.4 (−22.4 to 1.5) | −1.38 | 61.5 (33.7) | −5.2 (15.0 to 4.6) | −0.30 |
n = 4;
n = 5.
CES, couple-oriented education and support; CI, confidence interval; d, effect size, which was corrected for small sample size; PAP, positive airway pressure; PES, patient-oriented education and support; UC, usual care.
Sleep quality and daytime function
Means, change scores, and within-group effect sizes for PSQI, ESS, and FOSQ among patients are shown in Table 3. For the CES group, within-group effect sizes were large and negative for PSQI (d = −1.01) and medium and negative for ESS (d = −0.50) and FOSQ (d = 0.51). Large, negative effect size for PSQI (d = −0.94), small to medium, negative effect size for ESS (d = −0.42), and small to medium, positive effect size for FOSQ (d = 0.41) were observed for the PES group. For the UC group, within-group effect sizes were small to medium and negative for PSQI (d = −0.40), large and negative for ESS (d = −0.82), and medium and positive for FOSQ (d = 0.57). At 3-month follow-up, CES patients showed consistently robust improvements for all three outcomes.
Table 3.
Patient sleep and daytime function outcomes at baseline and 3 months after PAP therapy initiation.
| Baseline, Mean (SD) | 3 months, Mean (SD) | Change from baseline to 3 months, Mean (95% Cl) | d | |
|---|---|---|---|---|
| Pittsburgh Sleep Quality Index | ||||
| CES | 8.4 (2.5) | 5.0 (3.4)a | −4.3 (−8.4 to −0.1) | −1.01 |
| PES | 7.8 (3.1)b | 5.8 (3.7)b | −2.0 (−5.7 to 1.7) | −0.94 |
| UC | 8.3 (4.5) | 6.1 (5.8) | −2.2 (−5.0 to 0.6) | −0.40 |
| Epworth Sleepiness Scale | ||||
| CES | 9.0 (6.9) | 4.2 (0.8) | −4.8 (−10.3 to 0.7) | −0.51 |
| PES | 6.8 (6.8) | 3.3 (2.1) | −3.5 (−8.5 to 1.5) | −0.42 |
| UC | 10.8 (5.9) | 6.4 (4.8) | −4.3 (−8.4 to −0.2) | −0.82 |
| Functional Outcomes of Sleep Questionnaire | ||||
| CES | 16.6 (2.6)b | 17.9 (0.9) | 2.1 (−1.5 to 5.6) | 0.51 |
| PES | 17.2 (3.3) | 19.1 (1.5) | 1.9 (−1.0 to 4.8) | 0.41 |
| UC | 15.6 (4.3) | 17.7 (2.8) | 2.1 (−1.5 to 5.6) | 0.57 |
n = 4;
n=5.
CES, couple-oriented education and support; CI, confidence interval; d, effect size, which was corrected for small sample size; PAP, positive airway pressure; PES, patient-oriented education and support; UC, usual care.
Partner outcomes
Means, change scores, and within-group effect sizes for PSQI, ESS, and FOSQ among partners are shown in Table 5. For the CES group, within-group effect sizes were large and negative for ESS (d = −1.31), large and negative for FOSQ (d = 1.54), and small to medium and negative for PSQI (d = −0.31). Medium to large, positive effect size for PSQI (d = 0.65) and no change in ESS and FOSQ were observed for the PES group. For the UC group, within-group effect sizes were medium to large and negative for PSQI (d = −0.77) and ESS (d = −0.77) and medium to large and positive for FOSQ (d = 0.65).
Table 5.
Partner sleep and daytime function outcomes at baseline and 3 months after PAP therapy initiation.
| Baseline, Mean (SD) | 3 months, Mean (SD) | Change from baseline to 3 months, Mean (95% Cl) | d | |
|---|---|---|---|---|
| Pittsburgh Sleep Quality Index | ||||
| CES | 7.0 (2.9)a | 6.4 (5.0) | −1.5 (−5.5 to 2.5) | −0.31 |
| PES | 7.2 (2.1)b | 7.4 (3.7)b | 1.8 (−5.8 to 2.3) | 0.65 |
| UC | 9.9 (3.0)c | 7.3 (2.8)d | −2.7 (−6.0 to 0.6) | −0.77 |
| Epworth Sleepiness Scale | ||||
| CES | 3.8 (1.9) | 1.5 (2.5)a | −1.8 (−5.2 to 1.7) | −1.31 |
| PES | 6.5 (4.6) | 6.9 (4.5) | 0.4 (−2.4 to 3.2) | 0.08 |
| UC | 7.3 (5.2) | 4.7 (3.4) | −2.7 (−4.9 to −0.4) | −0.77 |
| Functional Outcomes of Sleep Questionnaire | ||||
| CES | 16.7 (2.6) | 18.2 (2.1) | 1.5 (0.4 to 2.7) | 1.54 |
| PES | 16.9 (3.9)b | 17.3 (2.9) | 0.0 (−1.2 to 1.2) | 0.00 |
| UC | 17.3 (2.4) | 18.0 (1.6) | 0.8 (−0.1 to 1.6) | 0.65 |
n = 4;
n = 5;
n = 7;
n = 8.
CES, couple-oriented education and support; CI, confidence interval; d, effect size, which was corrected for small sample size; PAP, positive airway pressure; PES, patient-oriented education and support; UC, usual care.
Discussion
The findings of this pilot study provide preliminary evidence that an education and support intervention targeting new PAP users and their partners is feasible and beneficial for both patients and partners. First, improvements in sleep quality, daytime sleepiness, and daytime function were found for both patients and partners in the CES group. However, it is important to note that, on average, patients and partners in the CES group had baseline ESS scores below the traditional cutoff of > 10, which indicates excessive daytime sleepiness (Johns, 1991). Second, patients in the CES group increased their PAP use over the first month of treatment; however, CPAP use declined back to 1-week values at 3-month follow-up. These findings suggest that the CES intervention may have short-term effects on PAP adherence, yet periodic booster sessions may be needed to sustain PAP use long-term (Bakker et al., 2016). Additionally, partners’ assistance and support that occurred in the early phase of treatment could have diminished over time, and thus, informing partners that their support may need to be long-term should be considered in the future. Lastly, all patients and partners in the CES group reported that the intervention improved their knowledge of sleep apnea and PAP treatment, and that it was helpful to see a demonstration of the PAP equipment, discuss PAP concerns, and attend sessions with their partner. All partners reported that the intervention helped them to identify ways to help their partner use the PAP. These findings are consistent with results from couples-oriented interventions in various chronic illnesses, suggesting a couples approach to disease management may be acceptable and beneficial for both patients and partners (Martire et al., 2010).
The recruitment rate in our study was 32%, which is lower than CPAP adherence interventions that are not couples-based (Aloia, Arnedt, Strand, Millman, & Borrelli, 2013; Bakker et al., 2016; Lai, Fong, Lam, Weaver, & Ip, 2014; Olsen, Smith, Oei, & Douglas, 2012). Attrition from the CES and PES interventions occurred prior to the first session. Once a couple completed the first session, they completed the remaining two sessions and the 3-month follow-up assessment, suggesting they were highly motivated to complete the intervention after initial engagement. Couples-oriented interventions face greater recruitment challenges, including requirement of two persons to consent and coordination of couples’ schedules to attend sessions together. Additionally, the need to travel to the study site may have been a potential barrier to participation. Future work will need to consider these barriers to participation and explore alternative delivery methods such as telemedicine to enhance recruitment efforts (Sparrow, Aloia, DeMolles, & Gottlieb, 2010).
Because this was a pilot study, the results are preliminary and will need replication in larger samples before efficacy of the CES intervention can be determined. A larger trial would enable examination of certain subgroups (e.g., men or women) and dyadic effects of the intervention. Longer-term follow-up is also needed, because it is possible that improvements in patients may precede and lead to improvements in partners. Another limitation of this pilot study is the use of a single respiratory therapist to deliver both the CES and PES interventions. Although the study protocol clearly defined which information and strategies were provided to each group, it is possible that discussions during the PES intervention could have become couples-focused.
Despite limitations, this study is the first to take a couples intervention approach to the problem of PAP adherence. Previous PAP adherence intervention studies allowed partners to be present during the sessions, but these interventions were not couples-oriented (Hoy, Vennelle, Kingshott, Engleman, & Douglas, 1999; Richards, Bartlett, Wong, Malouff, & Grunstein, 2007). There is increasing recognition of partners’ role in PAP adherence and data suggests that support and encouragement from partners is an important facilitator of PAP adherence (Baron, Gunn, Czajkowski, Smith, & Jones, 2012; Baron, Gunn, Wolfe, & Zee, 2017; Baron et al., 2011; Batool-Anwar, Baldwin, Fass, & Quan, 2017; Luyster, 2017; Ye et al., 2015). Accordingly, our findings indicate that a couples-oriented PAP adherence intervention aimed at providing education and support for PAP therapy helped partners identify ways to support patients’ PAP use, which quite likely contributed to increased PAP adherence during the first month of treatment, and improved sleep and daytime functioning for both patients and partners. A larger, adequately powered study is needed to more fully evaluate the effect of a couples approach on PAP adherence and patients’ and partners’ outcomes.
Acknowledgments
We would like to thank Anthony Wesolowski, Miranda Kuzman, Grace Pinto, Mary Kathryn Flood, Taylor Giambrone, Aminata Kamara, Rachel Klinges, Madeline Lepore, and Jennifer Mai.
Funding
This study was funded by the National Institutes of Health K23 HL1058870 and K02 AG039412.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/hbsm.
References
- Aloia MS, Arnedt JT, Riggs RL, Hecht J, & Borrelli B (2004). Clinical management of poor adherence toCPAP: Motivational enhancement. Behavioral Sleep Medicine, 2(4), 205–222. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Arnedt JT, Strand M, Millman RP, & Borrelli B (2013). Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep, 36(11), 1655–1662. doi: 10.5665/sleep.3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, … McEvoy RD. (2011). The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep, 34(1), 111–119. doi: 10.1093/sleep/34.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, … Patel SR. (2016). Motivational enhancement for increasing adherence to CPAP: A randomized controlled trial. Chest, 150(2), 337–345. doi: 10.1016/j.chest.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbé F, Durán-Cantolla J, Capote F, De La Peña M, Chiner E, Masa JF, … De Atauri JD. (2010). Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. American Journal of Respiratory and Critical Care Medicine, 181(7), 718–726. doi: 10.1164/rccm.200901-0050OC [DOI] [PubMed] [Google Scholar]
- Baron KG, Gunn HE, Czajkowski LA, Smith TW, & Jones CR (2012). Spousal involvement in CPAP: Does pressure help? Journal of Clinical Sleep Medicine, 8(2), 147–153. doi: 10.5664/jcsm.1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron KG, Gunn HE, Wolfe LF, & Zee PC (2017). Relationships and CPAP adherence among women with obstructive sleep apnea. Sleep Science and Practice, 1(1), 10. doi: 10.1186/s41606-017-0011-x [DOI] [Google Scholar]
- Baron KG, Smith TW, Berg CA, Czajkowski LA, Gunn H, & Jones CR (2011). Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep and Breathing, 15(3), 525–534. doi: 10.1007/s11325-010-0374-z [DOI] [PubMed] [Google Scholar]
- Batool-Anwar S, Baldwin CM, Fass S, & Quan SF (2017). Role of spousal involvement in continuous positive airway pressure (CPAP) adherence in patients with obstructive sleep apnea (OSA). Southwest Journal of Pulmonary & Critical Care, 14(5), 213–227. doi: 10.13175/swjpcc034-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CA, & Upchurch R (2007). A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychological Bulletin, 133(6), 920–954. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index:A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Chasens ER, Ratcliffe SJ, & Weaver TE (2009). Development of the FOSQ-10: A short version of the functional outcomes of sleep questionnaire. Sleep, 32(7), 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MR, Espie CA, Bartlett DJ, & Grunstein RR (2014). Integrating psychology and medicine in CPAP adherence—New concepts? Sleep Medicine Reviews, 18(2), 123–139. doi: 10.1016/j.smrv.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Engleman HM, & Wild MR (2003). Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Medicine Reviews, 7(1), 81–99. [DOI] [PubMed] [Google Scholar]
- Franks MM, Stephens MAP, Rook KS, Franklin BA, Keteyian SJ, & Artinian NT (2006). Spouses’ provision of health-related support and control to patients participating in cardiac rehabilitation. Journal of Family Psychology, 20(2), 311–318. [DOI] [PubMed] [Google Scholar]
- Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, & Cates CJ (2006). Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews, 2006(3), CD001106. [DOI] [PubMed] [Google Scholar]
- Hedges L, & Olkin I (1985). Statistical methods for meta-analysis. San Diego, CA: Academic Press. [Google Scholar]
- Helgeson VS, Novak SA, Lepore SJ, & Eton DT (2004). Spouse social control efforts: Relations to health behavior and well-being among men with prostate cancer. Journal of Social and Personal Relationships, 21(1), 53–68. [Google Scholar]
- Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, & Douglas NJ (1999). Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? American Journal of Respiratory and Critical Care Medicine, 159(4), 1096–1100. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Davies RJ, Mullins R, & Stradling JR (1999). Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised prospective parallel trial. Lancet, 353(9170), 2100–2105. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14(6),540–545. [DOI] [PubMed] [Google Scholar]
- Khan CM, Stephens MAP, Franks MM, Rook KS, & Salem JK (2013). Influences of spousal support and control on diabetes management through physical activity. Health Psychology, 32(7), 739–747. doi: 10.1037/a0028609 [DOI] [PubMed] [Google Scholar]
- Lai AY, Fong DY, Lam JC, Weaver TE, & Ip MS (2014). The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: A randomized controlled trial. Chest, 146(3), 600–610. doi: 10.1378/chest.13-2228 [DOI] [PubMed] [Google Scholar]
- Lewis MA, & Butterfield RM (2007). Social control in marital relationships: Effect of one’s partner on health behaviors. Journal of Applied Social Psychology, 37(2), 298–319. doi: 10.1111/j.0021-9029.2007.00161.x [DOI] [Google Scholar]
- Luyster F (2017). Impact of obstructive sleep apnea and its treatments on partners: A literature review. Journal of Clinical Sleep Medicine, 13(3), 467–477. doi: 10.5664/jcsm.6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster FS, Dunbar-Jacob J, Aloia MS, Martire LM, Buysse DJ, & Strollo PJ (2016). Patient and partner experiences with obstructive sleep apnea and CPAP treatment: A qualitative analysis. Behavioral Sleep Medicine, 14(1), 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire LM, Hemphill RC, & Polenick CA (2016). Harnessing the power of the marital relationship to improve illness management: Considerations for couple-based interventions In Bookwala J (Ed.), Couple relationships in the middle and later years: Their nature, complexity, and role in health and illness (pp. 325–344). Washington, DC: American Psychological Association. [Google Scholar]
- Martire LM, Schulz R, Helgeson VS, Small BJ, & Saghafi EM (2010). Review and meta-analysis of couple-oriented interventions for chronic illness. Annals of Behavioral Medicine, 40(3), 325–342. doi: 10.1007/s12160-010-9216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S, Smith SS, Oei TP, & Douglas J (2012). Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 80(1), 151–163. doi: 10.1037/a0026302 [DOI] [PubMed] [Google Scholar]
- Revenson TA, Kayser KE, & Bodenmann GE (2005). Couples coping with stress: Emerging perspectives on dyadic coping. New York, NY: American Psychological Association. [Google Scholar]
- Richards D, Bartlett DJ, Wong K, Malouff J, & Grunstein RR (2007). Increased adherence to CPAP with a group cognitive behavioral treatment intervention: A randomized trial. Sleep, 30(5), 635–640. doi: 10.1093/sleep/30.5.635 [DOI] [PubMed] [Google Scholar]
- Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, & Weaver TE (2011). A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews, 15(6), 343–356. doi: 10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow D, Aloia M, DeMolles DA, & Gottlieb DJ (2010). A telemedicine intervention to improve adherence to continuous positive airway pressure: A randomised controlled trial. Thorax, 65(12), 1061–1066. doi: 10.1136/thx.2009.133215 [DOI] [PubMed] [Google Scholar]
- Stephens MAP, Franks MM, Rook KS, Iida M, Hemphill RC, & Salem JK (2013). Spouses’ attempts to regulate day-to-day dietary adherence among patients with type 2 diabetes. Health Psychology, 32(10), 1029–1037. doi: 10.1037/a0030018 [DOI] [PubMed] [Google Scholar]
- Weaver TE, & Grunstein RR (2008). Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proceedings of the American Thoracic Society, 5(2), 173–178. doi: 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, … Pack A. (2007). Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep, 30(6), 711–719. doi: 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkove N, Baltzan M, Kamel H, Dabrusin R, & Palayew M (2008). Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Canadian Respiratory Journal, 15(7), 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DR, Lasserson TJ, & Smith I (2014). Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews, 2014(1), CD007736. doi: 10.1002/14651858.CD007736.pub2 [DOI] [PubMed] [Google Scholar]
- Ye L, Malhotra A, Kayser K, Willis DG, Horowitz JA, Aloia MS, & Weaver TE (2015). Spousal involvement and CPAP adherence: A dyadic perspective. Sleep Medicine Reviews, 19, 67–74. doi: 10.1016/j.smrv.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, & Aloia MS (2006). Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest, 130(6), 1772–1778. [DOI] [PubMed] [Google Scholar]