Abstract
The study was undertaken to find out the cause and etiology of an outbreak presumed to be due to water contamination that caused high morbidity in the western part of the state of Odisha during May, 2014. In this investigation 56 blood samples were collected and tested for HEV IgM through ELISA. Blood sample of 22 patients collected within 1 weeks post onset of symptoms and were subjected to RT-PCR and sequencing followed by phylogenetic analysis. Water samples were also analyzed for viral and bacterial contamination. A total of 290 individuals were examined for suspected jaundice. Out of 56 blood samples in 41 (73.2%) IgM for HEV was found. 12 samples out of 22 early phase samples were positive for RT-PCR and through phylogenetic analysis all were found to be of Genotype 1 and subtype A. This large outbreak was confirmed due to Hepatitis E virus and transmission was fast due to contamination of drinking water sources and lack of hygienic practices. The outcome of this investigation has created alertness among state health and municipal authorities to be prepared for the similar kind of situation for other part of the state.
Keywords: HEV, Outbreak, Odisha, Genotype 1A
Enterically transmitted hepatitis E virus (HEV) can cause both sporadic and epidemic illness in developing countries [3], where the disease is endemic because of poor sanitation and hygiene. It is estimated that it contributes 90% of all sporadic cases of acute viral hepatitis. Many outbreaks were reported from different parts of India [3, 10, 11]. It affects mainly young adults in 15–40 years age group [1] but remains as a subclinical infection in children [8]. Though infection is self limited, it has severe complication with high mortality of 20–30% in third trimester of pregnancy.
This present study is the first report of such a large outbreak of jaundice in the western part of Odisha along with molecular typing of the Hepatitis E virus.
Cases of Jaundice were reported from Sambalpur district situated in western part of the state of Odisha, Eastern India during the month of May, 2014. The city of Sambalpur has a population of 1,83,147 living in 29 wards and is spread over 33.66 sq.km. with a population density of 5441 per Sq. km. Sporadic cases were reported from May to November, 2014. During the month of December, a large number of people were affected and admitted to head quarter hospital and VSS Medical college, Burla, Sambalpur.
Information on the total number of members in the household, age, sex and drinking water source was obtained using a pre designed questionnaire. All the individuals (n = 290) having jaundice or past history of Jaundice in recent past were examined. Informed written consent was obtained from the agreed individuals who were examined and blood samples were collected from 56 symptomatic individuals. Patients complaining of acute jaundice were recorded from house hold survey as well as district health records. The criteria for diagnosis of hepatitis were defined as cases presenting with jaundice, dark coloured urine, fever of about 38 °C, elevation of alanine transaminase > 2.5 times normal and/or bilirubin > 1 mg/dl in serum and/or presence of bile salts and pigments in urine.
Blood samples were tested for Hepatitis E and Hepatitis A IgM antibody through ELISA by using commercially available kits (MP Biomedicals, Singapore & General Biologicals, Taiwan respectively). Out of the 56 blood samples, RT-PCR was performed on 22 samples those were collected within 1 week of symptom onset for detection of HEV RNA to amplify a region of RNA-dependent RNA polymerase (RdRp) gene of 325 bp. The RNA was extracted by using QIAGEN extraction kit (Quiagen, GmbH, Germany). PCR amplification steps were followed according to the protocol of Zhai et al. [14]. Briefly RNA was reverse transcribed using random primers (Promega, USA) and 200 units of Moloney murine leukaemia virus reverse transcriptase (Promega, USA) and Rnase Inhibitor (Invitrogen, Life Technologies, UK)to cDNA. Nested PCR was performed using the first round primers, MJC ESP 5-CATGGTAAAGTGGGTCAGGGTAT-3 and MJCEAP5-AGGGTGCCGGGCTCGCCGGA-3 followed by a second round of primers MJC ISP 5- GTATTTCGGCCTGGAGTAAGAC-3 and MJC IAP 5- TCACCGGAGTGYTTCTTCCAGAA-3. The amplified product was sequenced using ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, USA).
Phylogenetic analysis of nucleotide sequences was performed using the Bio Edit software (version 7.0.5.3). Dendrograms were constructed using MEGA (version 6.0), and genetic lineages were inferred by neighbour-joining algorithm using 1000 bootstrap replicates.
The quality of the water consumed by the population during the period was assessed. Municipal water supply from different locations were collected and tested for presumptive coliform count. The water samples were subjected to bacterial analysis by multiple fermentation tube method [13]). Water analysis was conducted on each of three serial dilutions (up to 10−3) separately for each water sample. The individual dilutions are used to inoculate tubes of culture medium which are then incubated at a standard temperature for a standard period of time. The presence of coliforms is indicated by turbidity in the culture medium and by a pH change and/or by the presence of gas.
Virological investigation was done in 30 l of water collected from 3 different locations (10 ltr each) at National Institute of Virology, Pune. The water samples were concentrated to 300 ml using membrane based ultra filtration technology which was followed by further concentration to 2 ml in an amicon cell (Millipore) and stored at − 70 °C in aliquots for PCR analysis [2].
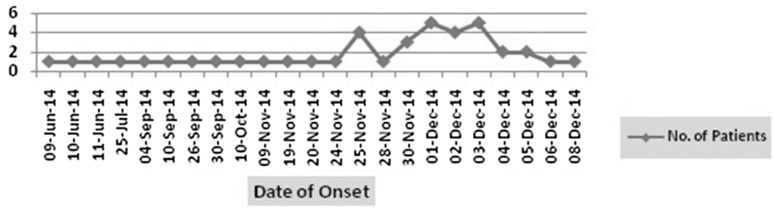
It was noted that during 2nd week of June the first case appeared and cases increased gradually towards last week of November 2014. Maximum number of cases were observed during 1st week of December and declined through 2nd week. As on 16th February 2015, a total of 2954 number of cases with 26 deaths reported from District Headquarter Hospital, Sambalpur, VSS MCH, Burla and Private nursing home at Sambalpur Municipality (DHS, Odisha). The survey included census which covered 4820 population residing in 290 households. A total of 290 individuals were examined to know the exact cause of Outbreak. The case attack rate was 1.6 and case fatality rate was 0.8 (2954 cases, 26 deaths for a population of 183,147). Attack rate was highest among 15–44 years and males were more affected than females. The day wise onset of cases was noted and the epicurve presented in Fig. 1.
Fig. 1.
Day wise onset of Jaundice cases from 9th June to 8th Dec’ 2014
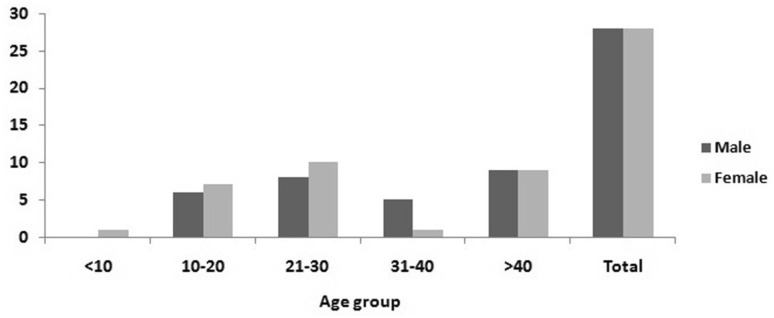
Among the 56 individuals, the cases were mostly from adults within the age range of 16–69 years. Only 4 were children < 15 years. Equal number of cases were recorded in 21–30 years and > 40 years of age group. Males and females were affected in different numbers in different age groups but over all males and females were equal in number (Fig. 2).
Fig. 2.
Age and sex distribution of affected cases
Most patients have mild to moderate grade of fever, yellowish discolouration of urine and sclera, anorexia, nausea, vomiting and severe weakness. There was no report of jaundice among pregnant women recorded.
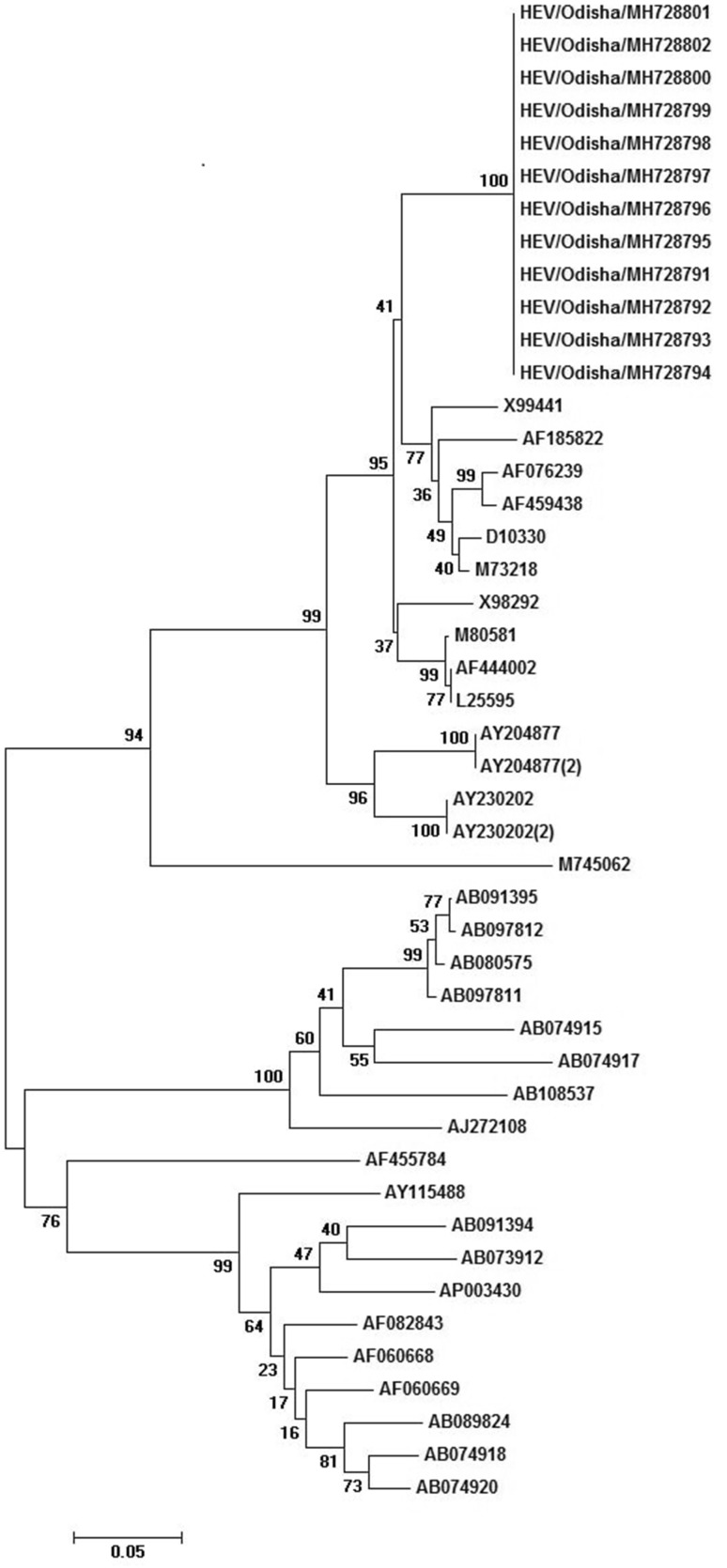
Of the 56 blood samples tested for HEV IgM antibodies, 41 (73.2%) were positive where as in one individual of 13 years male HAV antibody was detected. HEV RNA was detected in 12 no of cases in acute phase sample (n = 22) collected within 1 weeks post-onset of symptoms using RT-PCR. The sequence data shown that the strains belonged to Genotype 1, subtype A (Fig. 3). The sequences were almost similar among each other. The closest sequence to the present outbreak sequences was a strain isolated from a patient having fulminant hepatitis in India [4].
Fig. 3.
Phylogenetic tree constructed by neighbour-joining method by using MEGA 7 software. The nucleotide sequences of strains were isolated from Sambalpur district of Odisha. The accession number of strains are MH728791 to MH728802
Twelve water samples out of 24(50%) collected from different locations of the city were unsatisfactory with presumptive coliform counts > 1600/100 ml. None of the water samples tested for HEV RNA was found positive for viral RNA.
The present outbreak reported 2954 number of cases from June 2014 to Feb 2015. In India highest no of cases i.e. 79,000 were reported from Kanpur outbreak during 1991 [7]. During 2008, in Nellore district of Andhrapradesh, 23,915 cases of acute viral hepatitis was recorded (10). Apart from this frequent outbreaks were reported from many parts of the country [3, 11]. The case attack rate was 1.6 in comparison to 5.7 reported in Nellore district of Andhrapradesh which is the highest ever reported attack rate in the country [10]. Case attack rate is more in males than in females and young adults incomparison to children which is a similar kind of epidemiological feature reported by many authors [12] Case fatality rate is an important index to measure the extent of outbreak of a particular disease. Case fatality was varied as low as 0.22–12% in different outbreaks reported in comparison to our present case fatality rate i.e. 0.8% [10] A high case fatality rate (15–25%) among pregnant women is a characteristic hallmark of HEV infections being observed in many large epidemics reported worldwide. Miscarriages or premature delivery can also occur following HEV infection [6]. In the present epidemic there was no case of HEV infection among pregnant women in the survey population. This is contrary to reports from north India where a high incidence has been reported in pregnant women [9].
The sequences of strains isolated from Sambalpur is Genotype 1 and subtype A which is the predominant type circulating in India [3–5, 7]. The other closer strains were identified in different outbreaks reported from North India [5] and south India.
During the investigation it was observed that, the source of drinking water is pipe water supply from river through bigger overhead storage tanks and connected to individual houses through pipelines. In the areas, the water supply pipes are run by the side of drains carrying sewerage water. At many points the pipe line was leaking which allowed mixing of fecally contaminated drain water into domestic supply. Good personal hygiene especially hand washing practice was also not maintained and children were practicing open defecation. Drinking water filtration/boiling at house hold level was not adequate. The above observations point towards possible contamination of drinking water source as evident from high coliform count and improper personal hygiene (hand washing) that has caused the spread.
This study highlights Hepatitis E virus outbreak that occurred due to contamination of sewerage water with the domestic water supply. In a resource-limited state, particularly when urban areas grow rapidly, provision for sewerage disposal and protected water supply is often neglected, with serious consequences for public health which should be monitored regularly by the public health bodies. In absence of a vaccine and effective antiviral therapy, the source and supply of drinking water is the only way to control the outbreak. This outbreak has created alertness among state health and municipal authorities to be prepared for the similar kind of situation for other part of the state.
Acknowledgement
The authors would like to thank Indian Council of Medical Research for financial assistance, State public health department and National Institute of Virology, Pune for providing support during investigation.
References
- 1.Aggarwal R, Naik SR. Epidemiology of hepatitis E: past, present and future. Trop Gasteroenterol. 1997;18:49–56. [PubMed] [Google Scholar]
- 2.Arankalle VA, Sarada Devi KL, Lole KS, Shenoy KT, Verma V, Haneephabi M. Molecular characterization of hepatitis A virus from a large outbreak from Kerala, India. Indian J Med Res. 2006;123:760–769. [PubMed] [Google Scholar]
- 3.Arankalle VA, Chobe LP, Jha H, et al. Aetiology of acute sporadic non-A, non-B viral hepatitis in India. J Med Virol. 1993;40:121–125. doi: 10.1002/jmv.1890400208. [DOI] [PubMed] [Google Scholar]
- 4.Donati MC, Fagan EA, Harrison TJ. Sequence analysis of full length HEV clones directly derived from human liver in fulminant hepatitis E. In: Rizetto M, Purcell RH, Gerin JL, Verme G, editors. Viral hepatitis and liver disease. Torino: Edizoni Minerva Medica; 1997. pp. 313–316. [Google Scholar]
- 5.Jameel S, Zafrullah M, Chawla Y, Dilawari K, Jang B. Reevaluation of a North India isolate of hepatitis E virus based on the full-length genomic sequence obtained following long RT-PCR. Virus Res. 2002;86:53–58. doi: 10.1016/S0168-1702(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 6.Khuroo MS, Teli MR, Skidmore S, Sofia MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–255. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- 7.Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 8.Panda SK, Jameel S. Hepatitis E virus: from epidemiology to molecular biology. Viral Hepat Rev. 1997;3:227–251. [Google Scholar]
- 9.Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rosario V, Lalit N, Jeyaram I, Pawan KR, Rajiv S, Eapen CE, Kang G. Investigation of an epidemic of Hepatitis E in Nellore in south India. Trop Med Int Health. 2010;15:1333–1339. doi: 10.1111/j.1365-3156.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 11.Tandon BN, Joshi YK, Jain SK, et al. An epidemic of non-A, non-B hepatitis in north India. Indian J Med Res. 1982;75:739–744. [Google Scholar]
- 12.Tsega E, Hansson BG, Krawczynski K, Nordenfelt E. Acute sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin Infect Dis. 1992;14:961–965. doi: 10.1093/clinids/14.4.961. [DOI] [PubMed] [Google Scholar]
- 13.Bartram J, Ballance R, editors. Water quality monitoring- a preactical guide to the design and implementation of fresh water. Quality studies and monitoring programmes. Published on behalf of United Nations Environment Programme and the World Health Organization. © 1996 UNEP/WHO.
- 14.Zhai L, Dai X, Meng J. Hepatitis E virus genotyping based on full-length genome and partial genomic regions. Virus Res. 2006;120:57–69. doi: 10.1016/j.virusres.2006.01.013. [DOI] [PubMed] [Google Scholar]