Abstract
Mounting body of evidence indicates that the disruption of epithelial tight junctions and resulting loss of barrier function play a crucial role in the pathogenesis of a variety of gastrointestinal, hepatic, pulmonary, kidney and ocular diseases. Increased production of inflammatory mediators such as cytokines and reactive oxygen species disrupt the epithelial and endothelial barrier function by destabilizing tight junctions. Oxidative stress induced by various reactive oxygen species such as hydrogen peroxide, nitric oxide, peroxynitrite and hypochlorous acid disrupt the epithelial and endothelial tight junctions in various tissues. The mechanism involved in oxidative stress-induced disruption of tight junction includes protein modification such as thiol oxidation, phosphorylation, nitration and carbonylation. The role of signaling molecules such as protein kinases and protein phosphatases in regulation of tight junctions is discussed in this article. Understanding such mechanisms in oxidative stress-induced disruption of epithelial and endothelial barrier functions is likely to provide insight into the pathogenesis of various inflammatory diseases, and may form a basis for the design of treatment strategies for different diseases.
Keywords: hydrogen peroxide, nitric oxide, barrier function, epithelium, endothelium, occludin, tight junction, oxidative stress
2. INTRODUCTION
The epithelial tight junctions form a barrier to the entry of allergens, toxins and pathogens across the epithelium into the interstitial tissue in various organ systems, including the gastrointestinal tract, liver, lung and kidney. In the gastrointestinal tract, the disruption of tight junctions and the loss of epithelial barrier function increase the intestinal permeability to injurious factors leading to inflammation and mucosal injury. A significant body of evidence indicates that the disruption of tight junction and increase in paracellular permeability play a crucial role in the pathogenesis of gastrointestinal disorders, such as inflammatory bowel disease (IBD), alcoholic endotoxemia, infectious enterocolitis, celiac disease and necrotizing enterocolitis (NEC) (1–7). Elevated intestinal permeability to macromolecules was found in first-degree relatives of patients with IBD (8). Celiac disease is caused by hypersensitivity to dietary wheat gluten. Evidence indicates that wheat gluten disrupts the intestinal epithelial tight junctions and increase permeability to macromolecules (9). These observations suggest that the disruption of barrier function leading to epithelial paracellular permeability could be an initial step in the pathogenesis of IBD and other gastrointestinal disorders. Similarly, the disruption of epithelial tight junctions and accumulation of fluid and protein in the alveolar and bronchial space is involved in the pathogenesis of asthma (4) and acute respiratory distress syndrome (ARDS) (6). Nephrotic syndromes such as acquired glomerulopathies (10) and other kidney disorders (5) are also associated with a barrier dysfunction in glomerular and tubular epithelia. Disruption of tight junctions in corneal and retinal epithelia is involved in the pathogenesis of keratitis and retinopathy (7). The mechanism involved in the barrier dysfunction in different diseases is not well understood. However, a growing body of evidence indicates that a variety of inflammatory mediators such as cytokines and reactive oxygen species (ROS) disrupt the epithelial and endothelial barrier function in different tissues. In this article the regulation epithelial tight junctions and the barrier function by different members of ROS, especially hydrogen peroxide and nitric oxide is discussed. The current understanding of the mechanisms involved in ROS-induced disruption of tight junctions is summarized. This article addresses the tight junctions of epithelial tissues; however, the ROS-mediated regulation of endothelial barrier is also discussed for comparison. The focus of this article is to review the mechanism of ROS-induced barrier dysfunction, and therefore, the in vitro studies are discussed in greater detail.
3. TIGHT JUNCTIONS AND EPITHELIAL BARRIER FUNCTION
The epithelial barrier function is defined by the selective prevention of transport of solutes through the paracellular space on the basis of the size of the molecule and the charge it carries. Tight junctions, in general, selectively allow permeability to cations, while preventing the diffusion of anions (11). The solutes with a size less than 4.1 A° is freely permeable through the paracellular space, while the diffusion of solutes with size greater than 4.1 A° is not allowed. The tight junction is localized at the cell-cell contact sites at the apical end of epithelial cells (11). There are at least three types of transmembrane proteins, occludin, claudins and junction adhesion molecule, at the tight junction (12, 13). In addition to these transmembrane proteins, tight junctions associate with numerous peripheral proteins, which include ZO-1 (14), ZO-2 (15), ZO-3 (16), cingulin (17), 7H6 antigen (18), Rab3b (19), and symplekin (20). Occludin is the first transmembrane protein to be discovered. Although recent attention has been drawn toward the role of different isoforms of claudins in tight junction assembly, at present occludin is the most studied transmembrane protein of tight junctions.
3.1. Occludin
Occludin is a 65 kDa protein, which spans the plasma membrane four times to form two extracellular loops (46 and 48 amino acids) and one intracellular loop (10 amino acids). The short N-terminal tail (65 amino acids) and the long C-terminal tail (255 amino acids) extend into the intracellular compartment (12). The critical interactions between the extracellular loops have been attributed to the formation of barrier function of the tight junction (12). The intracellular tail of occludin interacts with the peripheral proteins, which in turn anchors tight junction protein complex to the actin cytoskeleton (21–23). Evidence indicates that interaction of C-terminal tail of occludin with peripheral proteins is crucial for the assembly of tight junction and the epithelial barrier function (24, 25). Limited information exists regarding the specific interactions among tight junction proteins. ZO-2 and ZO-3 interact with ZO-1, and it is likely to exist as ZO-1/ZO-2 and ZO-1/ZO-3 heterodimers (26). ZO proteins also interact directly with the C-terminal tail of occludin (27) and claudin 1–8 (28). All ZO proteins interact with cingulin, a tight junction plaque protein (17), and ZO-1 associates with the Ras-affecter molecule, AF-6 (29).
3.2. Association with the actin cytoskeleton
Actin filaments are associated with the cytoplasmic plaque of the tight junction (21, 22), and are believed to stabilize the tight junction assembly (30). Depolymerization of actin cytoskeleton leads to the disruption of tight junction (30). Although it was suggested that the C-terminal tail of occludin is linked to the actin cytoskeleton via ZO-1, ZO-2 and ZO-3, it was demonstrated that occludin can also interact directly with actin filaments (31). Rearrangement of actin cytoskeleton is an essential step in the reassembly of tight junctions after calcium depletion (32, 33). Disruption of tight junction by hydrogen peroxide involved the rearrangement of actin cytoskeleton (49, 50). Therefore, organization of actin cytoskeleton is essential for the assembly and the maintenance of tight junctions.
3.3. Regulation by signal transduction
Growing body evidence indicates that the activities of intracellular signaling molecules regulate the assembly and disassembly of tight junctions. Studies indicate that signaling pathways involving protein kinases, G-proteins, Rho/Rac GTPases are involved in the regulation of tight junction. Tyrosine kinases such as c-Yes, c-Src and focal adhesion kinase are localized at the vicinity of tight junction and adherens junction (34). A recent study demonstrated that c-Src plays an important role in the disruption of tight junctions (35), while c-Yes may be involved in the assembly of tight junction (36). Another study, by in vitro bait peptide pull down assay, showed that the regulatory subunit of phosphatidylinositol (PI) 3-kinase, p85, binds to the intracellular C-terminal region of occludin (37), raising the possibility that PI 3-kinase-dependent signaling pathway may be involved in the regulation of epithelial tight junctions. Inhibitors of PI 3-kinase prevent the VEGF-induced phosphorylation and redistribution of occludin and ZO-1 in bovine aortic endothelial cells (38). PI 3-kinase interacts with the C-terminal region of occludin and mediates the hydrogen peroxide induced activation of c-Src and the disruption of tight junctions in an intestinal epithelial monoloayer (39). Additionally, pharmacologic modulations of signaling molecules demonstrated that intracellular calcium (40), cyclic AMP (41), G-proteins (42–44), Rho GTPases (45) and protein kinases (35, 39, 46–52) regulate the integrity of epithelial tight junctions.
4. TIGHT JUNCTION DISRUPTION BY INFLAMMATORY MEDIATORS
Synthesis and secretion of a variety of inflammatory mediators are involved in the pathogenesis of inflammatory diseases in the gastrointestinal tract, lung, liver and kidney. Inflammatory cytokines and reactive oxygen species (ROS) play important role in the pathogenesis of inflammatory diseases. Attempts are being made to design therapeutic regimen for the treatment of many inflammatory diseases on the basis of attenuating the actions of inflammatory mediators. A growing body of evidence indicates that inflammatory mediators disrupt epithelial tight junctions. Oxidative stress induced by ROS such as hydrogen peroxide and nitric oxide (35, 46, 49, 52), cytokines such as TNFα, IFNγ and IL-6 (53–57), and proteases (58) disrupt tight junctions and increase paracellular permeability in a variety of epithelial tissues. Many of the gastrointestinal, lung, liver and kidney disorders are associated with oxidative stress (59–63), and the oxidative stress is well known to disrupt the epithelial tight junctions (64, 65). Therefore, it is important to understand the mechanisms involved in the ROS-induced disruption of tight junction, and its prevention by mucosal protective factors.
5. TIGHT JUNCTION DISRUPTION BY ROS
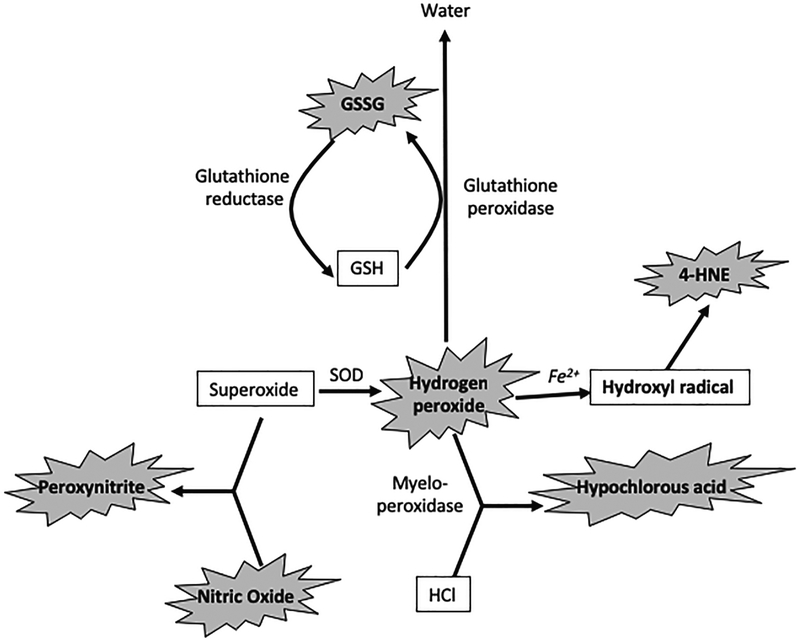
ROS, such as superoxide, hydrogen peroxide and hydroxyl radical are generated during the normal aerobic metabolism (66). Superoxide is generated by electron transport chain, NADPH oxidase, and xanthine oxidase activity (67). Superoxide undergoes dismutation reaction to generate hydrogen peroxide (68); this reaction is catalyzed by superoxide dismutase (SOD). Hydrogen peroxide is normally detoxified by antioxidant defense enzymes, such as catalase in peroxisomes and glutathione peroxidase in mitochondria and cytosol (68). However, hydrogen peroxide is also rapidly split to hydroxyl radical by Fenton reaction (67), which is catalyzed by transition metal ions, Fe2+ and Cu2+. Hydroxyl radical is considered more reactive than superoxide and hydrogen peroxide. Although all known ROS were found to be cytotoxic, little is known about the specific role of each ROS in oxidative stress-induced tissue injury. It is suggested that superoxide plays an important role in inflammatory response (69), and anti-inflammatory action of SOD was seen in several animal models of induced inflammation as well as in clinical trials in humans (70, 71). There is no evidence for the role of superoxide in lipid peroxidation, however, superoxide can reduce Fe3+ to Fe2+, which in turn may accelerate hydroxyl radical generation from hydrogen peroxide (Haber Weiss reaction) (72, 73). Additionally, superoxide can react with nitric oxide to generate peroxynitrite (74), which appears to be more toxic than superoxide. Therefore, it is believed that the toxicity of superoxide or hydrogen peroxide is mediated by their conversion into hydroxyl radical or peroxynitrite. Hydroxyl radical is the most reactive ROS, causing injury in every tissue. This oxidant species is suggested to be responsible for membrane lipid peroxidation (66), mitochondrial energization (66), hyaluronic acid degradation (75) and DNA fragmentation (76). Different ROS and relationships among them are illustrated in figure 1.
Figure 1:
Reactions generating different ROS. Components in bold represent different ROS that are potentially toxic to cells, while anti-oxidant components are in gray. Oxidants highlighted by explosion sign are known to disrupt the epithelial and endothelial barrier function. SOD, super oxide dismutase, GSH, glutathione, GSSG, oxidized glutathione, 4-HNE, 4-hydroxynonenol, HCl, hydrochloric acid.
ROS play an important role in ischemic tissue injury and pathogenesis of a number of intestinal disorders, including IBD and NEC (70, 77). Generation of ROS is elevated in patients with IBD, NEC, sclerosing cholangitis, alcoholic liver disease, ARDS and nephrotic syndromes (59, 61, 63, 70, 77–82). Additionally, ischemia-induced injury in various tissues is caused by generation of ROS and oxidative tissue injury. ROS are known to act directly on the cellular components causing lipid peroxidation, DNA fragmentation and cell necrosis. A significant body of evidence demonstrated that oxidative stress disrupts epithelial and/or endothelial tight junctions in the gastrointestinal tract (46, 47, 83, 84), liver (85), kidney (86), lung (87–90) and brain (91–93). Hydrogen peroxide and nitric oxide are the major members of ROS that are involved in tight junction disruption and barrier dysfunction. However, recent studies provided evidence to the role of peroxynitrite, hypochlorous acid and 4-hydroxy-2-nonenal (4-HNE) as key players in barrier dysfunction in epithelial and endothelial monolayers.
5.1. Hydrogen peroxide
Although it is well established that hydroxy radical is the major oxidant species involved in oxidative stress-induced cell injury, there is no evidence of tight junction regulation by this oxidant species. It is usually thought that most or all of the toxicity of superoxide and hydrogen peroxide involves their conversion to hydroxy radical or peroxynitrite. Very little is known about the specific role of hydrogen peroxide itself in tissue injury. Hydrogen peroxide at low micromolar levels is poorly reactive with biological systems (94). However, at higher concentrations, hydrogen peroxide can inactivate glyceraldehyde-3-phosphate dehydrogenase, a glycolytic enzyme (95). Ginsburg et al. (96) showed in kidney epithelial cell injury caused by xanthine oxidase in combination with a bacterial toxin was prevented by hydrogen peroxide scavengers, but not by SOD or deferoxamine, suggesting that hydrogen peroxide was the specific oxidant species that was responsible for cell injury in their model. Another study showed that hepatocyte injury by hydrogen peroxide was not affected by N,N’-diphenyl-p-phenylenediamine (a scavenger of lipid peroxides) suggesting that lipid peroxidation (usually caused by hydroxy radical) played no role in this cell injury. Hydroxy radical seems to play an important role in hydrogen peroxide-induced cell death in cultured gastric mucosal cells (96) and hepatocytes (97), but there is no evidence for its role in the initial disruption of tight junctions. Therefore, hydrogen peroxide and hydroxy radical may play distinct roles in the mechanism of ROS-induced cell injury. Hydrogen peroxide disrupts the barrier function in the colonic (35, 46, 47, 49, 52, 84, 87, 93, 98–102), airway (90, 103), tracheal (88, 104), alveolar (86, 105), retinal pigment epithelial (62) and renal tubular (87, 106, 107) epithelial monolayers. Hydrogen peroxide also induced barrier dysfunction in endothelial cell monolayers derived from brain microvasculature (91–93, 108–110), pulmonary artery and microvasculature (105, 108, 111–113) and human umbilical vein (108, 114).
In Caco-2 cell monolayers it was demonstrated that disruption of barrier function by xanthine oxidase activity was mediated by hydrogen peroxide; superoxide or hydroxy radical played no role in increasing the paracellular permeability in this model (47). Fe2+ inhibited hydrogen peroxide-induced paracellular permeability, suggesting a rapid conversion of hydrogen peroxide to hydroxyl radical by Fe2+ attenuates the effect of xanthine oxidase. In contrast, pretreatment of cell monolayer with deferoxamine or 1,10-phenanthroline (Fe chelators) resulted in a significant potentiation of hydrogen peroxide-induced increase in paracellular permeability. These observations clearly demonstrated that hydroxyl radical does not play a role in increase paracellular permeability in Caco-2 cell monolayer. This conclusion was further supported by the observation that vitamin E and vitamin A (hydroxyl radical scavengers) does not prevent hydrogen peroxide-induced permeability (47). Furthermore, administration of SOD slightly potentiated xanthine oxidase-induced permeability, suggesting that superoxide played no role in this disruption of barrier function. This was confirmed when hydrogen peroxide at concentration equivalent to that generated by xanthine oxidase also disrupted barrier function to a similar extent. Therefore, hydrogen peroxide is the cause of elevated paracellular permeability in Caco-2 cell monolayer.
A large number of studies have now demonstrated that hydrogen peroxide disrupts epithelial and endothelial barrier function leading to elevated paracellular permeability. Varying concentrations of hydrogen peroxide has been used to induce barrier dysfunction. While up to 100 mM hydrogen peroxide was used in some of the earlier studies (86) barrier dysfunction in Caco-2 cell monolayers was induced by 20 micromolar hydrogen peroxide (47). A long-range dose response curve showed a biphasic tight junction response to hydrogen peroxide in Caco-2 cell monolayers (Rao et al, unpublished). Hydrogen peroxide at 5–50 micromolar concentrations dose-dependently increased paracellular permeability, however this response was diminished at 100–500 micromolar concentrations. At 1–10 mM concentrations, hydrogen peroxide once again showed a dose-dependent increase in paracellular permeability. The mechanism involved in the biphasic response is unclear. However, this observation demonstrated that hydrogen peroxide at micromolar concentrations can disrupt the epithelial tight junctions and increase permeability to macromolecules. Although at high millimolar concentrations hydrogen peroxide is known to induce cell injury and apoptosis in several cell systems (115), it did not induce cell injury in Caco-2 cell monolayers at micromolar concentrations.
5.2. Nitric oxide
Nitric oxide is produced in every type of cells by converting L-arginine into citrulline by one of the three isoforms of nitric oxide synthase (NOS), eNOS, iNOS or nNOS (102, 116). While low basal level of nitric oxide is maintained in normal cells, a robust increase in NO by iNOS plays a crucial role in the inflammation-induced tissue injury (117). A few studies demonstrated that nitric oxide mediates lipopolysaccharide (LPS)-induced barrier dysfunction in the intestinal (118), hepatocellular (119) and pulmonary (119, 120) epithelial tissues. LPS-induced tight junction disruption in ileum and colon was attenuated by the administration of NOS inhibitor (118, 120). LPS failed to induce hepatocellular barrier disruption in iNOS knockout mice. Administration of iNOS inhibitor attenuated LPS-induced decrease in the expression of tight junction proteins and blood-to-bronchioalveolar flux of dextran in mice (120). Genetic deletion of iNOS in mice prevented dintrobenzylsulfonic acid-induced disruption of colonic epithelial and hepatocellular barrier (121). Several in vitro studies indicated that nitric oxide plays an important role in tight junction disruption in Sertoli (122), renal tubular (123) and lung (120) epithelial cell monolayers.
In contrast to the above observations, nitric oxide donors enhanced tight junction integrity in rat retinal epithelial pigment cell monolayers (102, 124, 125). Nitric oxide donors also attenuated tight junction disruption and paracellular permeability in gastric mucosa (125) and rat pigment epithelial cells (124). Therefore, nitric oxide produces opposing effects on tight junctions in different experimental conditions. It is not clear, at this point, what specific conditions are responsible for the discrepant findings of these studies. Certainly, the cell type and its differentiation states can be potential factors in the opposing responses to nitric oxide. However, the most likely explanation could be the concentration of nitric oxide (102). It is likely that low concentrations of nitric oxide enhances tight junction integrity and prevent disruption of tight junctions by injurious factors. On the other hand, high concentration of nitric oxide may be disruptive to tight junctions. Cyclic AMP, the downstream signal of nitric oxide, enhances tight junction integrity at 4–20 micromolar concentration, while it disrupts tight junctions at 100–500 micromolar concentration in Sertoli cell monolayers (102). Our recent studies demonstrated that nitric oxide donors at low concentration (up to 100 micromolar) enhances tight junction integrity, while at high dose (500 micromolar) it disrupts tight junction and increases paracellular permeability in rat bile duct epithelial cells (Seth et al, unpublished).
5.3. Peroxynitrite
As discussed above, there is no evidence of superoxide-induced influence on epithelial or endothelial barrier function. However, few studies indicated that nitric oxide reacts with superoxide to generate peroxynitrite, which is a highly toxic oxidant. Evidence suggests that peroxynitrite may induce a disruption of tight junction in epithelial and endothelial cell monolayers. Administration of peroxynitrite disrupts barrier function in Caco-2 cell monolayers (126, 127) and increases paracellular permeability and/or induce redistribution of ZO-1 in pulmonary (128) and umbilical (121) endothelial cell monolayers. Evidence also suggests that generation of peroxynitrite may be involved in TNFα-induced increase in permeability in pulmonary endothelial cells (127). Therefore, nitric oxide at low concentration may induce cell signaling to influence cell functions, while at higher concentration it may react with superoxide to produce peroxynitrite, which mediates the toxic effect of nitric oxide.
5.4. Hypochlorous acid
It is speculated that activated neutrophils produce hypochlorous acid, an extremely toxic oxidant. Recent studies indicated that hypochlorous acid increases permeability to ions and solutes in rabbit tracheal epithelium (129), and disrupts tight junctions and increase paracellular permeability in Caco-2 cell monolayers (98, 129–131).
5.5. 4-HNE
4-HNE is a biologically active aldehyde produced by membrane lipid peroxidation during the inflammation-induced oxidative stress, which may accumulate in tissues to a concentration as high as 5 mM (132). Few studies indicated that 4-HNE disrupts epithelial and endothelial tight junctions and the barrier function. 4-HNE induced lung endothelial barrier dysfunction by redox and MAP kinase-dependent mechanism (133, 134), and it also increased permeability of blood brain barrier (135) and human nasal epithelium (136).
6. MECHANISM OF TIGHT JUNCTION DISRUPTION BY ROS
6.1. Disruption of tight junction protein complex
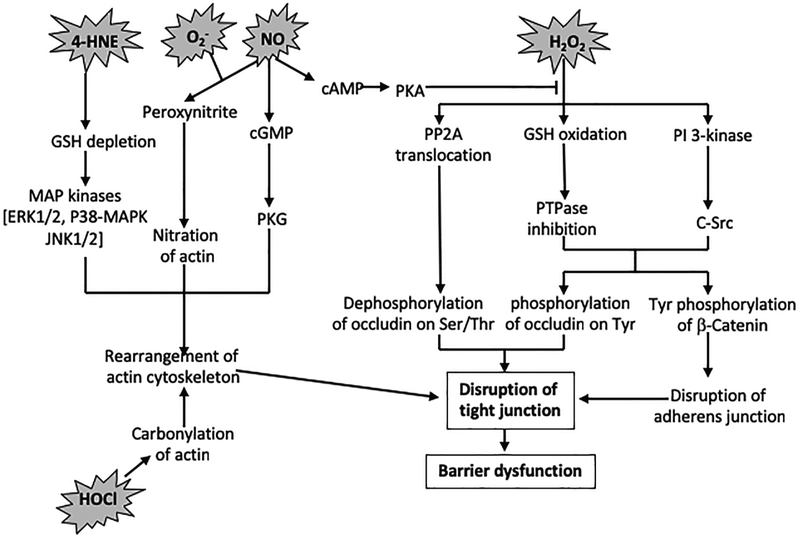
Many studies demonstrated that increase in epithelial paracellular permeability by various ROS is associated with a disruption of tight junctions and adherens junctions without affecting cell viability. Confocal immunofluorescence microscopy demonstrated that hydrogen peroxide, nitric oxide and 4-HNE all induced redistribution of tight junction proteins such as occludin and ZO-1 from the intercellular junctions into the intracellular compartment (35, 39, 49, 108, 114, 133, 134). This observation indicates that the interaction between ZO-1 and occludin is disrupted by oxidative stress. The interaction between occludin and ZO-1 at the intercellular junctions is crucial for the assembly and maintenance of epithelial tight junctions. Disruption of occludin-ZO-1 complex was further confirmed by a loss of co-immunoprecipitation of ZO-1 and occludin in hydrogen peroxide-treated cell monolayer (49). A significant body of evidence indicates that multiple mechanisms are involved in the ROS-induced disruption of tight junctions (Figure 2).
Figure 2:
Mechanisms involved in the epithelial barrier disruption by different ROS.
6.2. Disruption of adherens junctions
Adherens junctions, formed by interactions between E-cadherin and catenins, are localized just beneath the tight junctions. Although adherens junction does not form a physical barrier to the paracellular transport of macromolecules, it is known to indirectly influence the integrity of tight junctions. Disruption of adherens junctions by extra cellular calcium depletion was accompanied by disruption of tight junctions and increase in paracellular permeability (137). Hydrogen peroxide induced redistribution of E-cadherin and β-catenin from the intercellular junctions into the intracellular compartment and dissociated the interaction between E-cadherin and β-catenin in Caco-2 cell monolayers (49). Therefore, one mechanism by which ROS disrupt tight junctions may involve the disruption of adherens junctions.
6.3. Disruption of actin cytoskeleton and its interaction with the tight junction
ZO-1 establishes a link between occludin and cytoskeleton (31). Interaction with cytoskeleton anchors occludin at the tight junction, and this interaction is crucial for the maintenance of the structure and function of epithelial tight junction. Disruption of cytoskeleton by cytochalasin-D disrupts the tight junction and increases paracellular permeability (138). In Caco-2 cell monolayers it was shown that occludin and ZO-1 are associated with the cytoskeletal fraction of the cell. Hydrogen peroxide treatment completely depleted the occludin associated with the cytoskeletal fraction, and partially depleted cytoskeletal ZO-1. Hydrogen peroxide was also shown to disrupt the organization of actin cytoskeleton (139). ROS-induced disruption of barrier function in airway epithelial cell monolayers involved rearrangement of actin cytoskeleton (103). Hydrogen peroxide-induced increase in paracellular permeability was associated with a rapid dissociation of myosin heavy chain from actin. Rearrangement of actin cytoskeleton was also associated with the hydrogen peroxide-induced disruption of barrier function in brain (113, 122) and lung (114) microvascular endothelial monolayers. The mechanism involved in ROS-induced disruption of actin cytoskeleton is not clear. Covalent modification of G-actin and/or F-action may be involved.
6.4. Protein modification
The post-translational modifications proteins by thiol oxidation, phosphorylation, carbonylation and nitration may be involved in ROS-induced disruption of tight junctions. Oxidative stress-mediated protein thiol oxidation may result in modulation of functions of thiol containing proteins leading to activation of intracellular signaling pathways. Protein phosphorylation is known to regulate signaling molecules in a variety of cell systems. Oxidative stress may trigger several signaling pathways that mediate the tight junction disruption and barrier dysfunction. Nitration of key tyrosine residues and carbonylation of proteins may result in activation or inactivation of signaling proteins, or modulation of the dynamics of cytoskeleton. Therefore, protein modification may play a key role in altering intracellular signaling circuit, leading to disruption of tight junctions.
6.4.1. Protein thiol oxidation
Hydrogen peroxide in the cell is eliminated by glutathione (GSH)-mediated reduction by the antioxidant enzyme, glutathione peroxidase (68). However, excessive level of hydrogen peroxide results in GSH oxidation and accumulation of oxidized GSH (GSSG) (47). Reduction in GSH:GSSG ratio in the cell leads to oxidation of thiol proteins (140). The activation of redox-regulated molecules may trigger signaling pathways by hydrogen peroxide (141). The hydrogen peroxide-induced increase in paracellular permeability in Caco-2 cell monolayers was associated with the oxidation of GSH and protein thiols (47). Hydrogen peroxide-induced paracellular permeability was significantly inhibited by the pretreatment of cell monolayers with thiol compounds, such as GSH, N-acetyl-L-cysteine or dithiothreitol. Treatment with thiol compounds also showed increased levels of GSH and protein thiols in Caco-2 cell monolayer. This observation suggests that an elevation of intracellular thiols may protect the epithelium from hydrogen peroxide-induced cell injury, by protecting the cellular protein from thiol oxidation. Direct evidence of hydrogen peroxide-induced GSH oxidation in increasing paracellular permeability was provided by the effect of mercaptosuccinate, a GSH peroxidase inhibitor (142). Pretreatment of cell monolayers with mercaptosuccinate resulted in a concentration-related inhibition of hydrogen peroxide-induced increase in paracellular permeability, suggesting that GSH-peroxidase activity may be required for this effect of hydrogen peroxide. On the other hand, treatment with BCNU, an inhibitor of GSSG-reductase (143), potentiated hydrogen peroxide-induced increase in permeability. In contrast to GSH-peroxidase inhibitor, catalase inhibitor potentiated the effect of hydrogen peroxide on permeability. Inhibition of catalase may increase the intracellular hydrogen peroxide level and exacerbate the effect on permeability. Inhibition of GSH-peroxidase may also increase the intracellular hydrogen peroxide level; however, this hydrogen peroxide cannot oxidize GSH to GSSG in the absence of GSH-peroxidase activity. These findings supported the hypothesis that GSH oxidation and GSSG accumulation are crucial events in hydrogen peroxide-induced increase in permeability (47).
Treatment with hydrogen peroxide reduced the level of GSH and protein thiol in Caco-2 cells. Decrease in GSH was accompanied by an increase in GSSG level (47). The amount of increase in GSSG did not account for the amount of reduction in GSH. However, decrease in protein thiols did account for the decrease in GSH levels. This observation suggests that GSSG may rapidly react with protein thiols to form mixed disulfides. This effect of hydrogen peroxide on GSH oxidation was prevented by ferrous sulfate and potentiated by 1,10-phenanthroline (iron chelator). Mercaptosuccinate prevented hydrogen peroxide-induced oxidation of GSH and protein thiols, whereas BCNU potentiated this effect of hydrogen peroxide. Additionally, pretreatment with DEM (a sulfhydryl alkylator) inhibited hydrogen peroxide-induced decrease in transepithelial electrical resistance. DEM produced no significant effect on transepithelial electrical resistance in the absence of hydrogen peroxide, but it markedly reduced cellular GSH levels. However, DEM prevented hydrogen peroxide-induced generation of GSSG and depletion of protein thiols. These results suggested that generation of GSSG, rather than GSH depletion, is important in hydrogen peroxide-induced permeability. Accumulation of GSSG in hydrogen peroxide-treated cells was accompanied by reduced activity of cellular protein tyrosine phosphatase (PTPase) activity (47). PTPases are thiol enzymes with cysteine at the active site. Inhibition of PTPases may trigger tyrosine phosphorylation-dependent signaling pathways.
6.4.2. Protein phosphorylation
Hydrogen peroxide was previously shown to induce protein tyrosine phosphorylation in several cells (144–147). Although the significance of tyrosine phosphorylated proteins in mediating the biological effects of hydrogen peroxide is not known, a few studies (147–149) showed that protein tyrosine phosphorylation might play a role in the regulation of the integrity of adherens junctions and tight junctions of epithelial tissues. A decade ago we showed that hydrogen peroxide rapidly increases tyrosine phosphorylation of a wide spectrum of proteins in Caco-2 cells. It is likely that hydrogen peroxide stimulates tyrosine phosphorylation by GSSG-mediated inhibition of protein tyrosine phosphatases (PTPases). Some of the specific proteins that are tyrosine phosphorylated by hydrogen peroxide are occludin, ZO-1, E-cadherin and β-catenin, the tight junction and adherens junction proteins. Hydrogen peroxide induced protein tyrosine phosphorylation also in rat liver (85) and pulmonary (105) endothelial cells.
6.4.3. Nitration and carbonylation
A significant body of evidence indicates that nitric oxide and peroxynitrite-mediated nitration plays a crucial role in the cell signaling and tissue injury in various diseases (150, 151). Nitric oxide modulates cyclooxygenase and alters eicosanoid production, a crucial mechanism that may be involved in the pathogenesis of atherosclerosis (152). Nitration of tubulin in epithelial cells induced an alteration in cell morphology, change in microtubule organization and loss of epithelial barrier function (153). This was recently confirmed by other studies in intestinal epithelial monolayers (100, 154). Evidence of nitration of actin cytoskeleton and inhibition of actin polymerization by nitric-oxide-mediated actin nitration was demonstrated recently (155). Nitration of actin filaments was observed in colonic tissues from patients with IBD (156) and in Caco-2 cell monolayers treated with hydrogen peroxide (126, 157).
It is well established that protein carbonylation occur during oxidative stress due to modification by lipoperoxides and generation of hypochlorous acid. Protein carbonylation is implied to play a crucial role in the pathogenesis of various human diseases (158, 159). It was demonstrated that carbonylation of actin results in altered dynamics of actin cytoskeleton and disruption of epithelial barrier function (160). This was further supported by the observation that actin carbonylation is associated with hypochlorous acid-induced barrier dysfunction in an intestinal epithelial monolayer (130, 131).
6.5. Modulation of intracellular signal transduction
Hydrogen peroxide and nitric oxide activate a variety of intracellular signaling molecules. Hydrogen peroxide activates protein kinases such as c-Src, protein kinase C (PKC) and MAP kinases. Nitric oxide induces the production of cyclic AMP and cyclic GMP and activates protein kinase A (PKA) and protein kinase G (PKG), respectively. ROS-mediated protein modifications, as discussed above, may be involved in triggering the activation of these signaling molecules. The activation of signaling pathways is likely to mediate the ROS-induced disruption of tight junctions and barrier function in both epithelial and endothelial tissues. While the signaling mechanisms involved in NO-induced disruption of endothelial tight junctions are studied more recently, the mechanisms involved in hydrogen peroxide-induced disruption of epithelial tight junctions have been studied in much greater detail.
6.5.1. Protein tyrosine kinases and phosphatases
The relationship between hydrogen peroxide-induced protein tyrosine phosphorylation and paracellular permeability was determined by evaluating the effect of PTPase inhibitors, phenylarsine oxide (PAO) and vanadate, on basal epithelial permeability and the effect of tyrosine kinase inhibitor (genistein) on hydrogen peroxide-induced increase in epithelial permeability in Caco-2 cell monolayers (46). Both PAO and vanadate reduced the transepithelial electrical resistance and dilution potential and increased mannitol permeability. PAO and vanadate-induced increase in paracellular permeability was associated with an increase in protein tyrosine phosphorylation of numerous proteins. The electrophoretic profile of tyrosine-phosphorylated proteins was similar to that in hydrogen peroxide-treated cells. At low concentration (0.1 mM), vanadate induced a strong potentiation of the effect of hydrogen peroxide on paracellular permeability and protein tyrosine phosphorylation (46). All these observations indicate that there is a clear association between the increase in paracellular permeability and the protein tyrosine phosphorylation.
The hydrogen peroxide-induced increase in paracellular permeability in Caco-2 cell monolayer was accompanied by a partial decrease in protein tyrosine phosphatase (PTPase) activity (47). Interestingly, the PTPase activity in soluble fractions of Caco-2 cells was also inhibited by in vitro incubation with GSSG, suggesting that GSSG may directly interact with PTPases. Inhibition of PTPases may contribute to the protein tyrosine phosphorylation induced by hydrogen peroxide. These results indicate that elevation of intracellular GSSG is required for the hydrogen peroxide-induced increase in protein tyrosine phosphorylation and paracellular permeability. The level of GSSG attained may be regulated by the ratio of the activities of GSH-peroxidase and GSSG-reductase. The activity of GSH-peroxidase in the Caco-2 cell is 35-fold greater than the activity of GSSG-reductase (161). Elevated GSSG caused by GSH-peroxidase-dependent hydrogen peroxide metabolism may mediate protein thiol oxidation in the cell. The protein thiol oxidation by hydrogen peroxide may result in the inhibition of PTPases and/or activation of protein tyrosine kinases in Caco-2 cells. This view was further supported by other studies demonstrating that hydrogen peroxide activates tyrosine kinase (145) and inhibits PTPase (162). The inhibition of PTPases may involve oxidation of cysteine in the signature motif sequence at the active site of PTPases (163). GSH and other thiol compounds prevent the GSSG-mediated protein thiol oxidation and preserve PTPase activity and attenuate the hydrogen peroxide-induced increase in paracellular permeability.
Tyrosine kinase activity appears to be required for both the disassembly (46–49, 101, 149) and the assembly (36, 107, 164) of tight junctions. Tyrosine kinase inhibitors prevent hydrogen peroxide-induced disruption of tight junction in Caco-2 and T84 cell monolayers (46, 47, 49, 101). The hydrogen peroxide-induced decrease in transepithelial electrical resistance, increase in inulin permeability and redistribution of occludin and ZO-1 from the intercellular junctions were prevented by PP2, a selective inhibitor of Src kinases. This was associated with a rapid membrane translocation and activation of c-Src (35). Over expression of wild type or constitutively active c-Src enhanced the hydrogen peroxide-induced tyrosine phosphorylation of occludin, ZO-1 and β-catenin, disruption of tight junctions and increase in paracellular permeability in Caco-2 cell monolayers. In contrast, expression of inactive c-SrcK297R mutant attenuated oxidative stress-induced disruption of tight junction and increase in permeability (19, 35). The rates of recovery of resistance, increase in barrier to inulin, and reorganization of occludin and ZO-1 into the intercellular junctions during the calcium-induced reassembly of tight junction were much greater in Caco-2 cells transfected with c-SrcK297R as compared to those in cells transfected with empty vector or wild type c-Src. These studies demonstrated that c-Src plays a crucial role in oxidative stress-induced disruption of tight junction in Caco-2 cell monolayers.
Oxidative stress has been shown to activate Src family kinases in embryonic fibroblasts and Xenopus egg, while c-Src activity was reduced by oxidative stress in mesangial cells. The role of Src family kinases in oxidative stress-mediated alteration of cell functions was shown in embryonic cells and endothelial cells by using selective inhibitors of Src family kinases (discussed in ref. 33). The activity of Src family kinases was also shown in hydrogen peroxide-induced increase in endothelial permeability (165). In contrast to these effects, Src kinase activity was shown to be required for the assembly of tight junction in canine kidney epithelial cells (107). Similarly, another study indicated that c-Yes is associated with tight junctions in MDCK cell monolayers and play a role in the assembly of tight junction (36). Therefore, different members of Src family kinases may play different roles in the regulation of tight junction integrity.
Oxidative stress resulted in dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes and loss of their association with the cytoskeleton by a tyrosine kinase-dependent mechanism (49). The role of tyrosine kinase in the dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes indicates that tyrosine phosphorylation plays a role in dissociation of these protein complexes. This view was supported by the observation that hydrogen peroxide rapidly increased tyrosine phosphorylation of the tight junction and adherens junction proteins such as occludin, ZO-1, E-cadherin and β-catenin (48, 49). Therefore, tyrosine phosphorylation-dependent dissociation protein-protein interactions may play a crucial role in hydrogen peroxide-induced disruption of tight junctions and adherens junctions.
The in vitro phosphorylation of recombinant C-terminal region of occludin by c-Src and pull down assay for its binding to ZO-1 demonstrated that c-Src binds to C-terminal region of occludin and phosphorylates occludin on tyrosine residues (166). Tyrosine phosphorylation of occludin C-terminal region induced by c-Src resulted in a dramatic reduction in its affinity for binding to ZO-1 and ZO-3 (166). The localization of c-Src at the tight junction suggests a possible role of c-Src in tyrosine phosphorylation of occludin and its dissociation from ZO-1 and ZO-3. Similarly, our in vitro studies demonstrated that tyrosine phosphorylation of β-catenin results in the loss of its interaction with E-cadherin (167), suggesting that hydrogen peroxide-induced tyrosine phosphorylation of β-catenin may be involved in the disruption of adherens junction.
6.5.2. Protein Serine/threonine kinases and phosphatases
Occludin is hyper phosphorylated serine and threonine residues in the resting epithelium (168, 169). Assembly and disassembly of tight junctions are associated with altered phosphorylation of occludin on serine and threonine residues in MDCK cells (168–170), indicating that phosphorylation of occludin on serine and threonine residues plays a crucial role in the regulation of tight junction integrity. Phosphorylation of occludin may be mediated by atypical protein kinase C (PKC), such as PKCζ and PKCλ, which are localized in the vicinity of tight junction (171). A recent study showed that PP2A and PP1, the serine/threonine-phosphatases, interact with the tight junction protein complex and alter occludin phosphorylation and the integrity of tight junction in Caco-2 and MDCK cell monolayers (20, 137). Therefore, the balance between atypical PKC and PP2A may determine the serine and threonine phosphorylation status of occludin. PP2A and PP1 was shown to directly interact with occludin and dephosphorylate it on threonine and serine residues, respectively (137). Knock down of PP2A and PP1 by siRNA enhanced the integrity of tight junctions and accelerated the calcium-induced reassembly of tight junctions (137). Phosphorylation of occludin on serine and threonine residues was dramatically reduced during the disassembly of tight junction and threonine phosphorylation was gradually increased during reassembly. Our recent studies showed that occludin undergoes rapid dephosphorylation on threonine residues, but not serine residues, during the hydrogen peroxide-induced disruption of tight junction in Caco-2 cell monolayers (Rao et al unpublished); this was associated with a rapid increase in co-immunoprecipitation of PP2A with occludin. PP2A may induce dephosphorylation of occludin on threonine residues, leading to the disruption of tight junctions.
PKC activity is required for hydrogen peroxide-induced disruption of tight junctions and barrier dysfunction in human tracheal epithelial cells and intestinal epithelial monolayers (88, 172, 173). Hydrogen peroxide activated PKCδ and PKCλ, and knock down of these PKC isoforms attenuated the hydrogen peroxide-induced barrier dysfunction (88, 172, 173). However, the precise role of these PKC isoforms in tight junction disruption is unknown. On the other hand, PKC activity was not required for oxidative stress-induced disruption of barrier function in kidney tubular epithelial cells (174). PKC activity however was required for EGF-mediated protection of tight junctions from hydrogen peroxide (98). Therefore, the role of PKC in the disruption of tight junction by oxidative stress is not clear. The influence on tight junction integrity may depend on the PKC isoforms involved.
Although PKC, PKA and PKG play important role in regulation of microvascular permeability (175) there is no evidence reported so far for the role of PKC isoforms in the ROS-induced barrier dysfunction in endothelial cell monolayers. Nitric oxide triggers the production of second messengers such as cyclic AMP and cyclic GMP (123). Cyclic AMP and cyclic GMP are known to activate their downstream signals PKA and PKG, respectively. Therefore, it is likely that PKA and PKG play roles in nitric oxide-induced barrier dysfunction. Cyclic GMP was found to increase the permeability in MDCK cell monolayers (102, 176, 177), while cyclic AMP protects the tight junction against radiation-induced disassembly of tight junctions (65). PKA was also shown to play a role in nitric oxide-mediated protection of tight junction (65).
6.5.3. Role of PI 3-kinase
A previous study, by in vitro bait peptide pull down assay, showed that the regulatory subunit of PI 3-kinase, p85, binds to the intracellular C-terminal region of occludin (37), raising the possibility that PI 3-kinase-dependent signaling pathway plays a role in the regulation of tight junction integrity. PI 3-kinase activity is required for the VEGF-induced serine/threonine-phosphorylation and redistribution of occludin and ZO-1 in bovine aortic endothelial cells (38). Similarly, PI 3-kinase was found to interact with the tight junction and play a role in the regulation of hydrogen peroxide-induced epithelial permeability in an intestinal epithelial monolayer (39). Hydrogen peroxide rapidly increased the association of PI 3-kinase with occludin and induced translocation of PI 3-kinase to the intercellular junction. PI 3-kinase inhibitors attenuated the hydrogen peroxide-induced tyrosine phosphorylation and dissociation from the actin cytoskeleton of tight junction proteins, and therefore prevented the disruption of tight junctions. PI 3-kinae inhibitor also attenuated hydrogen peroxide-induced activation of c-Src, suggesting that hydrogen peroxide-mediated activation of PI 3-kinase is upstream to Src activation, which in turn induces tyrosine phosphorylation of tight junction and adherens junction proteins. The mechanism involved in hydrogen peroxide-induced activation of PI 3-kinase is not clear at this point. Further studies are required to determine the mechanisms involved in hydrogen peroxide-induced PI 3-kinase activation and PI 3-kinase-mediated activation of c-Src.
6.5.4. Role of MAP kinases
MAP kinases play important roles in hydrogen peroxide-induced disruption of barrier function in endothelial cell monolayers. Activation of ERK1/2 seem to play a major role in the oxidative stress-induced barrier dysfunction in brain endothelial monolayers (91, 108, 110). P38-MAP kinase is involved in hydrogen-peroxide-induced barrier dysfunction in human umbilical venous endothelial and pulmonary artery endothelial cell monolayers (113, 114). In umbilical endothelial cell monolayers, hydrogen peroxide-induced barrier dysfunction also requires the activity of ERK1/2 (108). Therefore, there is a clear agreement between all the studies in endothelial monolayers that activation of MAP kinases, ERK1/2, p38-MAPK and JNK1/2, is involved in the disruption of tight junctions and resulting barrier dysfunction. On the other hand, contrasting observations are made in epithelial monolayers.
There is only limited information available on the role of MAP kinases in the oxidative stress-induced disruption of barrier function in epithelial monolayers. A solitary study showed that p38-MAPK is involved in hydrogen peroxide-induced tight junction disruption in human colonic epithelia monolayers (84). Activation of ERK1/2 pathway by the expression of active Raf-1 leading in salivary gland epithelial monolayer (178) or transformation by active Ras in MDCK cells (179) resulted in disruption of tight junctions. Ras-induced transformation of MDCK cells prevents the assembly of tight junction by decreasing the levels of occludin (179), while Raf-1 in salivary gland epithelial cells decreases the expression of claudin-1 (178). Furthermore, the role of MAP kinase in tight junction disruption was shown in human corneal epithelial cell monolayers (180). However, a recent study showed that ERK1/2 activity is involved in EGF-mediated preservation of tight junction integrity from hydrogen peroxide (52). MAP kinase inhibitors showed no influence on hydrogen peroxide-induced permeability. The reason for such opposing effects of MAP kinase in epithelia from different origin is not clear. However, the level of basal activity of MAP kinases and/or their sub cellular localization may determine the type of influence on the tight junction. In Ras-transformed MDCK cells the active ERK was localized exclusively in the detergent-soluble fractions (179), while in Caco-2 cells, the active ERK was localized predominantly in the detergent-insoluble fraction of Caco-2 cells (52).
Co-localization of p-ERK with occludin suggested a possible interaction of p-ERK with the tight junction-protein complex. Co-immunoprecipitation of p-ERK with occludin and co-immunoprecipitation of occludin with p-ERK indicated that p-ERK does interact with the tight junction-protein complex, and this interaction is rapidly increased by EGF administration (52). This was further confirmed by occludin pull down assay. Incubation of recombinant fused C-terminal tail of occludin with protein extracts prepared from control and EGF-treated cells showed that p-ERK binds to the C-terminal sequence of occludin. The binding was greater in proteins extracted from EGF-treated cells, as there was higher levels of p-ERK present in these extracts compared to protein extracts prepared from the control cells. The precise mechanism involved in regulation of tight junctions is not clear. The EGF-mediated prevention of occludin dephosphorylation in hydrogen peroxide-treated cells was dependent on MAP kinase activity. MAP kinase activity either directly affect the threonine phosphorylation of occludin or it may modulate the activities of protein kinases and phosphatases involved in the regulation of occludin phosphorylation. On the other hand, cytosolic ERK may modulate the transcription machinery to attenuate the expression of tight junction proteins.
7. SUMMARY AND PERSPECTIVE
The current understanding of the mechanism of oxidative stress-induced barrier dysfunction in epithelial and endothelial cell monolayers include, the role of ROS members such as hydrogen peroxide and nitric oxide in the tight junction disruption and barrier dysfunction. Other reactive molecules such as 4-HNE and hypochlorous acid are emerging as potential players in oxidative stress-induced tissue injury. A significant body of evidence indicates that disassembly of actin cytoskeleton is an important event in ROS-induced barrier dysfunction, while activation of signaling molecules such as tyrosine kinases, PKC and MAP kinases may directly or indirectly target the tight junction protein complex, leading to disruption of barrier function. Overall, the current knowledge in this area indicates that ROS modulate multiple signaling pathways and engage multiple mechanisms to effectively disrupt the tight junctions and adherens junctions to break the epithelial and endothelial barrier. It is critical to dissect out these mechanisms and establish the link between ROS and tight junctions. Therefore, it is expected that the future studies will address the mechanisms involved in ROS-induced rearrangement of cytoskeleton, the modulation of protein kinases and phosphatases and their consequence on the assembly and maintenance of tight junction protein complex. Additionally, it is also crucial to understand the mechanisms of prevention of ROS-induced barrier dysfunction by protective factors such as EGF. Such protective factors may prove useful in designing the therapeutic strategy for the treatment of a variety of inflammatory diseases of lung, kidney, liver, eye and the gastrointestinal tract.
8. ACKNOWLEDGEMENTS
A portion of the studies discussed in this article and the review process are supported by NIH grants, DK55532, AA12307 and a grant from Morgan Foundation for Research on Primary Sclerosing Cholangitis.
Abbreviations:
- ROS
reactive oxygen species
- IBD
inflammatory bowel disease
- NEC
necrotizing engterocolitis
- ZO-1,2,3,
zonula occludens-1,2,3
- TNFα
transforming growth factor-alpha
- IFNγ
interferon-gamma
- IL-6
interleukin-6
- SOD
superoxide dismutase
- ARDS
acute respiratory distress syndrome
- 4-HNE
4-hydroxy nonenal
- NOS
nitric oxide synthase
- LPS
lipopolysaccharide
- GSH
glutathione
- GSSG
glutathione disulfide
- PKC
protein kinase C
- PKA
protein kinase A
- PKG
protein kinase G
- PAo
phenylarsine oxide
- PTPase
protein tyrosine phosphatase
- PP2A
protein phosphatase 2A
- PP1
protein phosphatase 1
9. REFERENCES
- 1.Laukoetter MG, Bruewer M and Nusrat A: Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol, 22(2), 85–9 (2006) [DOI] [PubMed] [Google Scholar]
- 2.Pravda J: Radical induction theory of ulcerative colitis. World J Gastroenterol, 11(16), 2371–84 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberhuber G and Vogelsang H: Gastrointestinal permeability in celiac disease. Gastroenterology, 114(1), 226 (1998) [DOI] [PubMed] [Google Scholar]
- 4.Godfrey RW: Human airway epithelial tight junctions. Microsc Res Tech, 38(5), 488–99 (1997) [DOI] [PubMed] [Google Scholar]
- 5.Lee DB, Huang E and Ward HJ: Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol, 290(1), F20–34 (2006) [DOI] [PubMed] [Google Scholar]
- 6.Budinger GR and Sznajder JI: The alveolar-epithelial barrier: a target for potential therapy. Clin Chest Med, 27(4), 655–69; abstract ix (2006) [DOI] [PubMed] [Google Scholar]
- 7.Erickson KK, Sundstrom JM and Antonetti DA: Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis, 10(2), 103–17 (2007) [DOI] [PubMed] [Google Scholar]
- 8.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T and Rotter JI: Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med, 105(6), 883–5 (1986) [DOI] [PubMed] [Google Scholar]
- 9.Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D’Agate C, Not T, Zampini L, Catassi C and Fasano A: Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol, 41(4), 408–19 (2006) [DOI] [PubMed] [Google Scholar]
- 10.Akhtar M and Al Mana H: Molecular basis of proteinuria. Adv Anat Pathol, 11(6), 304–9 (2004) [DOI] [PubMed] [Google Scholar]
- 11.Van Itallie CM and Anderson JM: Claudins and epithelial paracellular transport. Annu Rev Physiol, 68, 403–29 (2006) [DOI] [PubMed] [Google Scholar]
- 12.Tsukita S and Furuse M: Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol, 9(7), 268–73 (1999) [DOI] [PubMed] [Google Scholar]
- 13.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D and Dejana E: Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol, 142(1), 117–27 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson BR, Siliciano JD, Mooseker MS and Goodenough DA: Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol, 103(3), 755–66 (1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesaitis LA and Goodenough DA: Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol, 124(6), 949–61 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskins J, Gu L, Wittchen ES, Hibbard J and Stevenson BR: ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol, 141(1), 199–208 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D and Citi S: Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol, 147(7), 1569–82 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K and Mori M: Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol, 120(2), 477–83 (1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber E, Berta G, Tousson A, St John P, Green MW, Gopalokrishnan U, Jilling T, Sorscher EJ, Elton TS, Abrahamson DR and et al. : Expression and polarized targeting of a rab3 isoform in epithelial cells. J Cell Biol, 125(3), 583–94 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keon BH, Schafer S, Kuhn C, Grund C and Franke WW: Symplekin, a novel type of tight junction plaque protein. J Cell Biol, 134(4), 1003–18 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madara JL: Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol, 253(1 Pt 1), C171–5 (1987) [DOI] [PubMed] [Google Scholar]
- 22.Fanning AS, Jameson BJ, Jesaitis LA and Anderson JM: The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem, 273(45), 29745–53 (1998) [DOI] [PubMed] [Google Scholar]
- 23.Itoh M, Nagafuchi A, Moroi S and Tsukita S: Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol, 138(1), 181–92 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M and Matter K: Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol, 134(4), 1031–49 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T and Tsukita S: Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol, 141(2), 397–408 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittchen ES, Haskins J and Stevenson BR: Exogenous expression of the amino-terminal half of the tight junction protein ZO-3 perturbs junctional complex assembly. J Cell Biol, 151(4), 825–36 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S and Tsukita S: Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol, 127(6 Pt 1), 1617–26 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh M, Furuse M, Morita K, Kubota K, Saitou M and Tsukita S: Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol, 147(6), 1351–63 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C and Kaibuchi K: The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol, 139(3), 785–95 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madara JL, Barenberg D and Carlson S: Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol, 102(6), 2125–36 (1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittchen ES, Haskins J and Stevenson BR: Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem, 274(49), 35179–85 (1999) [DOI] [PubMed] [Google Scholar]
- 32.Ivanov AI, Hunt D, Utech M, Nusrat A and Parkos CA: Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell, 16(6), 2636–50 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP and Braga VM: Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci, 118(Pt 23), 5549–62 (2005) [DOI] [PubMed] [Google Scholar]
- 34.Anderson JM and Van Itallie CM: Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol, 269(4 Pt 1), G467–75 (1995) [DOI] [PubMed] [Google Scholar]
- 35.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM and Rao RK: Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem, 278(14), 11916–24 (2003) [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Lu Q, Goodenough DA and Jeansonne B: Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell, 13(4), 1227–37 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C and Mrsny RJ: The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem, 275(38), 29816–22 (2000) [DOI] [PubMed] [Google Scholar]
- 38.Pedram A, Razandi M and Levin ER: Deciphering vascular endothelial cell growth factor/vascular permeability factor signaling to vascular permeability. Inhibition by atrial natriuretic peptide. J Biol Chem, 277(46), 44385–98 (2002) [DOI] [PubMed] [Google Scholar]
- 39.Sheth P, Basuroy S, Li C, Naren AP and Rao RK: Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem, 278(49), 49239–45 (2003) [DOI] [PubMed] [Google Scholar]
- 40.Lacaz-Vieira F and Marques MM: Pulses of cell Ca(2+) and the dynamics of tight junction opening and closing. J Membr Biol, 196(2), 117–27 (2003) [DOI] [PubMed] [Google Scholar]
- 41.Bijlsma PB, Bakker R and Groot JA: The chloride conductance of tight junctions of rat ileum can be increased by cAMP but not by carbachol. J Membr Biol, 157(2), 127–37 (1997) [DOI] [PubMed] [Google Scholar]
- 42.Ries J, Stein J, Traynor-Kaplan AE and Barrett KE: Dual role for AlF4(−)-sensitive G proteins in the function of T84 epithelial cells: transport and barrier effects. Am J Physiol, 272(3 Pt 1), C794–803 (1997) [DOI] [PubMed] [Google Scholar]
- 43.Denker BM, Saha C, Khawaja S and Nigam SK: Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J Biol Chem, 271(42), 25750–3 (1996) [DOI] [PubMed] [Google Scholar]
- 44.Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA and Nusrat A: Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology, 121(3), 566–79 (2001) [DOI] [PubMed] [Google Scholar]
- 45.Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P and Madara JL: Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci U S A, 92(23), 10629–33 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao RK, Baker RD, Baker SS, Gupta A and Holycross M: Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol, 273(4 Pt 1), G812–23 (1997) [DOI] [PubMed] [Google Scholar]
- 47.Rao RK, Li L, Baker RD, Baker SS and Gupta A: Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol, 279(2), G332–40 (2000) [DOI] [PubMed] [Google Scholar]
- 48.Atkinson KJ and Rao RK: Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol, 280(6), G1280–8 (2001) [DOI] [PubMed] [Google Scholar]
- 49.Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr and Gupta A: Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J, 368(Pt 2), 471–81 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ and Banan A: The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther, 316(1), 1–7 (2006) [DOI] [PubMed] [Google Scholar]
- 51.Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB and Madara JL: PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol, 277(3 Pt 1), C554–62 (1999) [DOI] [PubMed] [Google Scholar]
- 52.Basuroy S, Seth A, Elias B, Naren AP and Rao R: MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J, 393(Pt 1), 69–77 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner JR: Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol, 169(6), 1901–9 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA and Nusrat A: Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell, 16(10), 5040–52 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA and Nusrat A: Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J, 19(8), 923–33 (2005) [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL and Fink MP: IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol, 285(3), G621–9 (2003) [DOI] [PubMed] [Google Scholar]
- 57.Tazuke Y, Drongowski RA, Teitelbaum DH and Coran AG: Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int, 19(5), 321–5 (2003) [DOI] [PubMed] [Google Scholar]
- 58.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL and Egleton RD: Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia, 50(1), 202–11 (2007) [DOI] [PubMed] [Google Scholar]
- 59.Rezaie A, Parker RD and Abdollahi M: Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci, 52(9), 2015–21 (2007) [DOI] [PubMed] [Google Scholar]
- 60.Dani C, Cecchi A and Bertini G: Role of oxidative stress as physiopathologic factor in the preterm infant. Minerva Pediatr, 56(4), 381–94 (2004) [PubMed] [Google Scholar]
- 61.Riccioni G, Barbara M, Bucciarelli T, di Ilio C and D’Orazio N: Antioxidant vitamin supplementation in asthma. Ann Clin Lab Sci, 37(1), 96–101 (2007) [PubMed] [Google Scholar]
- 62.Zhang H, Slutsky AS and Vincent JL: Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Med, 26(4), 474–6 (2000) [DOI] [PubMed] [Google Scholar]
- 63.Harada K and Nakanuma Y: Molecular mechanisms of cholangiopathy in primary biliary cirrhosis. Med Mol Morphol, 39(2), 55–61 (2006) [DOI] [PubMed] [Google Scholar]
- 64.Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ and Cheetham ME: Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci, 45(2), 675–84 (2004) [DOI] [PubMed] [Google Scholar]
- 65.Perez LM, Milkiewicz P, Ahmed-Choudhury J, Elias E, Ochoa JE, Sanchez Pozzi EJ, Coleman R and Roma MG: Oxidative stress induces actin-cytoskeletal and tight-junctional alterations in hepatocytes by a Ca2+ -dependent, PKC-mediated mechanism: protective effect of PKA. Free Radic Biol Med, 40(11), 2005–17 (2006) [DOI] [PubMed] [Google Scholar]
- 66.Farber JL: Mechanisms of cell injury by activated oxygen species. Environ Health Perspect, 102 Suppl 10, 17–24 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halliwell B: Mechanisms involved in the generation of free radicals. Pathol Biol (Paris), 44(1), 6–13 (1996) [PubMed] [Google Scholar]
- 68.Halliwell B, Clement MV and Long LH: Hydrogen peroxide in the human body. FEBS Lett, 486(1), 10–3 (2000) [DOI] [PubMed] [Google Scholar]
- 69.Halliwell B: Superoxide, iron, vascular endothelium and reperfusion injury. Free Radic Res Commun, 5(6), 315–8 (1989) [DOI] [PubMed] [Google Scholar]
- 70.Emerit J, Pelletier S, Tosoni-Verlignue D and Mollet M: Phase II trial of copper zinc superoxide dismutase (CuZnSOD) in treatment of Crohn’s disease. Free Radic Biol Med, 7(2), 145–9 (1989) [DOI] [PubMed] [Google Scholar]
- 71.Miller MJ, McNeill H, Mullane KM, Caravella SJ and Clark DA: SOD prevents damage and attenuates eicosanoid release in a rabbit model of necrotizing enterocolitis. Am J Physiol, 255(5 Pt 1), G556–65 (1988) [DOI] [PubMed] [Google Scholar]
- 72.Halliwell B: Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett, 96(2), 238–42 (1978) [DOI] [PubMed] [Google Scholar]
- 73.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD and Beckman JS: Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys, 298(2), 431–7 (1992) [DOI] [PubMed] [Google Scholar]
- 74.Ischiropoulos H, Zhu L and Beckman JS: Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys, 298(2), 446–51 (1992) [DOI] [PubMed] [Google Scholar]
- 75.Del Maestro R, Thaw HH, Bjork J, Planker M and Arfors KE: Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl, 492, 43–57 (1980) [PubMed] [Google Scholar]
- 76.Halliwell B and Aruoma OI: DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett, 281(1–2), 9–19 (1991) [DOI] [PubMed] [Google Scholar]
- 77.Clark DA, Fornabaio DM, McNeill H, Mullane KM, Caravella SJ and Miller MJ: Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am J Pathol, 130(3), 537–42 (1988) [PMC free article] [PubMed] [Google Scholar]
- 78.Louie S, Halliwell B and Cross CE: Adult respiratory distress syndrome: a radical perspective. Adv Pharmacol, 38, 457–90 (1997) [DOI] [PubMed] [Google Scholar]
- 79.Halliwell B and Cross CE: Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect, 102 Suppl 10, 5–12 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halliwell B: Oxidative stress and cancer: have we moved forward? Biochem J, 401(1), 1–11 (2007) [DOI] [PubMed] [Google Scholar]
- 81.Greene EL and Paller MS: Oxygen free radicals in acute renal failure. Miner Electrolyte Metab, 17(2), 124–32 (1991) [PubMed] [Google Scholar]
- 82.Stratta P, Canavese C, Dogliani M, Mazzucco G, Monga G and Vercellone A: The role of free radicals in the progression of renal disease. Am J Kidney Dis, 17(5 Suppl 1), 33–7 (1991) [PubMed] [Google Scholar]
- 83.Fink MP: Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care, 9(2), 143–51 (2003) [DOI] [PubMed] [Google Scholar]
- 84.Oshima T, Sasaki M, Kataoka H, Miwa H, Takeuchi T and Joh T: Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell Mol Life Sci (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadari YR, Geiger B, Nadiv O, Sabanay I, Roberts CT Jr., LeRoith D and Zick Y: Hepatic tyrosine-phosphorylated proteins identified and localized following in vivo inhibition of protein tyrosine phosphatases: effects of H2O2 and vanadate administration into rat livers. Mol Cell Endocrinol, 97(1–2), 9–17 (1993) [DOI] [PubMed] [Google Scholar]
- 86.Welsh MJ, Shasby DM and Husted RM: Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest, 76(3), 1155–68 (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim KJ and Suh DJ: Asymmetric effects of H2O2 on alveolar epithelial barrier properties. Am J Physiol, 264(3 Pt 1), L308–15 (1993) [DOI] [PubMed] [Google Scholar]
- 88.Yamaya M, Sekizawa K, Masuda T, Morikawa M, Sawai T and Sasaki H: Oxidants affect permeability and repair of the cultured human tracheal epithelium. Am J Physiol, 268(2 Pt 1), L284–93 (1995) [DOI] [PubMed] [Google Scholar]
- 89.Gardner SY, Brody AR, Mangum JB and Everitt JI: Chrysotile asbestos and H2O2 increase permeability of alveolar epithelium. Exp Lung Res, 23(1), 1–16 (1997) [DOI] [PubMed] [Google Scholar]
- 90.Waters CM, Savla U and Panos RJ: KGF prevents hydrogen peroxide-induced increases in airway epithelial cell permeability. Am J Physiol, 272(4 Pt 1), L681–9 (1997) [DOI] [PubMed] [Google Scholar]
- 91.Fischer S, Wiesnet M, Renz D and Schaper W: H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol, 84(7), 687–97 (2005) [DOI] [PubMed] [Google Scholar]
- 92.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB and Kim KY: Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res, 68(3), 231–8 (2004) [DOI] [PubMed] [Google Scholar]
- 93.Yamagata K, Tagami M, Takenaga F, Yamori Y and Itoh S: Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis, 17(3), 491–9 (2004) [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B: The chemistry of free radicals. Toxicol Ind Health, 9(1–2), 1–21 (1993) [DOI] [PubMed] [Google Scholar]
- 95.McKenzie SJ, Baker MS, Buffinton GD and Doe WF: Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest, 98(1), 136–41 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ginsburg I, Misgav R, Pinson A, Varani J, Ward PA and Kohen R: Synergism among oxidants, proteinases, phospholipases, microbial hemolysins, cationic proteins, and cytokines. Inflammation, 16(5), 519–38 (1992) [DOI] [PubMed] [Google Scholar]
- 97.Starke PE and Farber JL: Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem, 260(18), 10099–104 (1985) [PubMed] [Google Scholar]
- 98.Banan A, Choudhary S, Zhang Y, Fields JZ and Keshavarzian A: Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radic Biol Med, 28(5), 727–38 (2000) [DOI] [PubMed] [Google Scholar]
- 99.Katsube T, Tsuji H and Onoda M: Nitric oxide attenuates hydrogen peroxide-induced barrier disruption and protein tyrosine phosphorylation in monolayers of intestinal epithelial cell. Biochim Biophys Acta, 1773(6), 794–803 (2007) [DOI] [PubMed] [Google Scholar]
- 100.Banan A, Zhang L, Fields JZ, Farhadi A, Talmage DA and Keshavarzian A: PKC-zeta prevents oxidant-induced iNOS upregulation and protects the microtubules and gut barrier integrity. Am J Physiol Gastrointest Liver Physiol, 283(4), G909–22 (2002) [DOI] [PubMed] [Google Scholar]
- 101.Rao R, Baker RD and Baker SS: Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol, 57(6), 685–95 (1999) [DOI] [PubMed] [Google Scholar]
- 102.Lee NP and Cheng CY: Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol Reprod, 70(2), 267–76 (2004) [DOI] [PubMed] [Google Scholar]
- 103.Boardman KC, Aryal AM, Miller WM and Waters CM: Actin re-distribution in response to hydrogen peroxide in airway epithelial cells. J Cell Physiol, 199(1), 57–66 (2004) [DOI] [PubMed] [Google Scholar]
- 104.Ohrui T, Yamaya M, Sekizawa K, Yamada N, Suzuki T, Terajima M, Okinaga S and Sasaki H: Effects of rhinovirus infection on hydrogen peroxide- induced alterations of barrier function in the cultured human tracheal epithelium. Am J Respir Crit Care Med, 158(1), 241–8 (1998) [DOI] [PubMed] [Google Scholar]
- 105.Carbajal JM and Schaeffer RC Jr.: H2O2 and genistein differentially modulate protein tyrosine phosphorylation, endothelial morphology, and monolayer barrier function. Biochem Biophys Res Commun, 249(2), 461–6 (1998) [DOI] [PubMed] [Google Scholar]
- 106.Jepson MA: Disruption of epithelial barrier function by H2O2: distinct responses of Caco-2 and Madin-Darby canine kidney (MDCK) strains. Cell Mol Biol (Noisy-le-grand), 49(1), 101–12 (2003) [PubMed] [Google Scholar]
- 107.Meyer TN, Schwesinger C, Ye J, Denker BM and Nigam SK: Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem, 276(25), 22048–55 (2001) [DOI] [PubMed] [Google Scholar]
- 108.Kevil CG, Oshima T, Alexander B, Coe LL and Alexander JS: H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol, 279(1), C21–30 (2000) [DOI] [PubMed] [Google Scholar]
- 109.Haorah J, Knipe B, Gorantla S, Zheng J and Persidsky Y: Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem, 100(2), 324–36 (2007) [DOI] [PubMed] [Google Scholar]
- 110.Krizbai IA and Deli MA: Signalling pathways regulating the tight junction permeability in the blood-brain barrier. Cell Mol Biol (Noisy-le-grand), 49(1), 23–31 (2003) [PubMed] [Google Scholar]
- 111.Seeger W, Hansen T, Rossig R, Schmehl T, Schutte H, Kramer HJ, Walmrath D, Weissmann N, Grimminger F and Suttorp N: Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability--effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res, 50(1), 1–17 (1995) [DOI] [PubMed] [Google Scholar]
- 112.Gupta MP, Steinberg HO and Hart CM: H2O2 causes endothelial barrier dysfunction without disrupting the arginine-nitric oxide pathway. Am J Physiol, 274(4 Pt 1), L508–16 (1998) [DOI] [PubMed] [Google Scholar]
- 113.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL and Natarajan V: Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal, 5(6), 723–30 (2003) [DOI] [PubMed] [Google Scholar]
- 114.Kevil CG, Oshima T and Alexander JS: The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium, 8(2), 107–16 (2001) [DOI] [PubMed] [Google Scholar]
- 115.Jin GF, Hurst JS and Godley BF: Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr Eye Res, 22(3), 165–73 (2001) [DOI] [PubMed] [Google Scholar]
- 116.Bogdan C: Nitric oxide and the regulation of gene expression. Trends Cell Biol, 11(2), 66–75 (2001) [DOI] [PubMed] [Google Scholar]
- 117.Grisham MB, Jourd’Heuil D and Wink DA: Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites:implications in inflammation. Am J Physiol, 276(2 Pt 1), G315–21 (1999) [DOI] [PubMed] [Google Scholar]
- 118.Han X, Fink MP, Yang R and Delude RL: Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock, 21(3), 261–70 (2004) [DOI] [PubMed] [Google Scholar]
- 119.Han X, Fink MP, Uchiyama T, Yang R and Delude RL: Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol, 286(1), G126–36 (2004) [DOI] [PubMed] [Google Scholar]
- 120.Han X, Fink MP, Uchiyama T, Yang R and Delude RL: Increased iNOS activity is essential for pulmonary epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Lung Cell Mol Physiol, 286(2), L259–67 (2004) [DOI] [PubMed] [Google Scholar]
- 121.Mazzon E, De Sarro A, Caputi AP and Cuzzocrea S: Role of tight junction derangement in the endothelial dysfunction elicited by exogenous and endogenous peroxynitrite and poly(ADP-ribose) synthetase. Shock, 18(5), 434–9 (2002) [DOI] [PubMed] [Google Scholar]
- 122.Lee NP and Cheng CY: Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3’,5’-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology, 144(7), 3114–29 (2003) [DOI] [PubMed] [Google Scholar]
- 123.Somosy Z, Bognar G, Horvath G and Koteles GJ: Role of nitric oxide, cAMP and cGMP in the radiation induced changes of tight junctions in Madin-Darby canine kidney cells. Cell Mol Biol (Noisy-le-grand), 49(1), 59–63 (2003) [PubMed] [Google Scholar]
- 124.Zech JC, Pouvreau I, Cotinet A, Goureau O, Le Varlet B and de Kozak Y: Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci, 39(9), 1600–8 (1998) [PubMed] [Google Scholar]
- 125.Kwiecien S, Brzozowski T, Konturek P and Konturek SJ: The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J Physiol Pharmacol, 53(4 Pt 2), 761–73 (2002) [PubMed] [Google Scholar]
- 126.Banan A, Fields JZ, Zhang Y and Keshavarzian A: iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am J Physiol Gastrointest Liver Physiol, 280(6), G1234–46 (2001) [DOI] [PubMed] [Google Scholar]
- 127.Neumann P, Gertzberg N, Vaughan E, Weisbrot J, Woodburn R, Lambert W and Johnson A: Peroxynitrite mediates TNF-alpha-induced endothelial barrier dysfunction and nitration of actin. Am J Physiol Lung Cell Mol Physiol, 290(4), L674–L684 (2006) [DOI] [PubMed] [Google Scholar]
- 128.Knepler JL Jr., Taher LN, Gupta MP, Patterson C, Pavalko F, Ober MD and Hart CM: Peroxynitrite causes endothelial cell monolayer barrier dysfunction. Am J Physiol Cell Physiol, 281(3), C1064–75 (2001) [DOI] [PubMed] [Google Scholar]
- 129.Guo Y, Krumwiede M, White JG and Wangensteen OD: HOCl effects on the tight junctions of rabbit tracheal epithelium. Am J Physiol, 270(2 Pt 1), L224–31 (1996) [DOI] [PubMed] [Google Scholar]
- 130.Banan A, Zhang Y, Losurdo J and Keshavarzian A: Carbonylation and disassembly of the F-actin cytoskeleton in oxidant induced barrier dysfunction and its prevention by epidermal growth factor and transforming growth factor alpha in a human colonic cell line. Gut, 46(6), 830–7 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Banan A, Fitzpatrick L, Zhang Y and Keshavarzian A: OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic Biol Med, 30(3), 287–98 (2001) [DOI] [PubMed] [Google Scholar]
- 132.Parola M, Bellomo G, Robino G, Barrera G and Dianzani MU: 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal, 1(3), 255–84 (1999) [DOI] [PubMed] [Google Scholar]
- 133.Usatyuk PV and Natarajan V: Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem, 279(12), 11789–97 (2004) [DOI] [PubMed] [Google Scholar]
- 134.Usatyuk PV, Parinandi NL and Natarajan V: Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem, 281(46), 35554–66 (2006) [DOI] [PubMed] [Google Scholar]
- 135.Mertsch K, Blasig I and Grune T: 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett, 314(3), 135–8 (2001) [DOI] [PubMed] [Google Scholar]
- 136.Koizumi J, Kojima T, Kamekura R, Kurose M, Harimaya A, Murata M, Osanai M, Chiba H, Himi T and Sawada N: Changes of Gap and Tight Junctions during Differentiation of Human Nasal Epithelial Cells Using Primary Human Nasal Epithelial Cells and Primary Human Nasal Fibroblast Cells in a Noncontact Coculture System. J Membr Biol, 218(1–3), 1–7 (2007) [DOI] [PubMed] [Google Scholar]
- 137.Seth A, Sheth P, Elias BC and Rao R: Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem, 282(15), 11487–98 (2007) [DOI] [PubMed] [Google Scholar]
- 138.Ma TY, Hollander D, Tran LT, Nguyen D, Hoa N and Bhalla D: Cytoskeletal regulation of Caco-2 intestinal monolayer paracellular permeability. J Cell Physiol, 164(3), 533–45 (1995) [DOI] [PubMed] [Google Scholar]
- 139.Banan A, Fields JZ, Zhang Y and Keshavarzian A: Phospholipase C-gamma inhibition prevents EGF protection of intestinal cytoskeleton and barrier against oxidants. Am J Physiol Gastrointest Liver Physiol, 281(2), G412–23 (2001) [DOI] [PubMed] [Google Scholar]
- 140.O’Donovan DJ and Fernandes CJ: Mitochondrial glutathione and oxidative stress: implications for pulmonary oxygen toxicity in premature infants. Mol Genet Metab, 71(1–2), 352–8 (2000) [DOI] [PubMed] [Google Scholar]
- 141.Droge W, Schulze-Osthoff K, Mihm S, Galter D, Schenk H, Eck HP, Roth S and Gmunder H: Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J, 8(14), 1131–8 (1994) [PubMed] [Google Scholar]
- 142.Storey BT: Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod, 3(3), 203–13 (1997) [DOI] [PubMed] [Google Scholar]
- 143.Walther UI, Czermak A, Muckter H, Walther SC and Fichtl B: Decreased GSSG reductase activity enhances cellular zinc toxicity in three human lung cell lines. Arch Toxicol, 77(3), 131–7 (2003) [DOI] [PubMed] [Google Scholar]
- 144.Heffetz D, Bushkin I, Dror R and Zick Y: The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem, 265(5), 2896–902 (1990) [PubMed] [Google Scholar]
- 145.Hayes GR and Lockwood DH: Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci U S A, 84(22), 8115–9 (1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koshio O, Akanuma Y and Kasuga M: Hydrogen peroxide stimulates tyrosine phosphorylation of the insulin receptor and its tyrosine kinase activity in intact cells. Biochem J, 250(1), 95–101 (1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Collares-Buzato CB, Jepson MA, McEwan GT, Simmons NL and Hirst BH: Junctional uvomorulin/E-cadherin and phosphotyrosine-modified protein content are correlated with paracellular permeability in Madin-Darby canine kidney (MDCK) epithelia. Histochemistry, 101(3), 185–94 (1994) [DOI] [PubMed] [Google Scholar]
- 148.Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A and Geiger B: The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J, 11(5), 1733–42 (1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staddon JM, Herrenknecht K, Smales C and Rubin LL: Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci, 108 ( Pt 2), 609–19 (1995) [DOI] [PubMed] [Google Scholar]
- 150.Sabetkar M, Low SY, Naseem KM and Bruckdorfer KR: The nitration of proteins in platelets: significance in platelet function. Free Radic Biol Med, 33(6), 728–36 (2002) [DOI] [PubMed] [Google Scholar]
- 151.Ceriello A: Nitrotyrosine: new findings as a marker of postprandial oxidative stress. Int J Clin Pract Suppl(129), 51–8 (2002) [PubMed] [Google Scholar]
- 152.Upmacis RK, Deeb RS and Hajjar DP: Oxidative alterations of cyclooxygenase during atherogenesis. Prostaglandins Other Lipid Mediat, 80(1–2), 1–14 (2006) [DOI] [PubMed] [Google Scholar]
- 153.Eiserich JP, Estevez AG, Bamberg TV, Ye YZ, Chumley PH, Beckman JS and Freeman BA: Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci U S A, 96(11), 6365–70 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Banan A, Fields JZ, Decker H, Zhang Y and Keshavarzian A: Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther, 294(3), 997–1008 (2000) [PubMed] [Google Scholar]
- 155.Clements MK, Siemsen DW, Swain SD, Hanson AJ, Nelson-Overton LK, Rohn TT and Quinn MT: Inhibition of actin polymerization by peroxynitrite modulates neutrophil functional responses. J Leukoc Biol, 73(3), 344–55 (2003) [DOI] [PubMed] [Google Scholar]
- 156.Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y and Fields JZ: Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut, 52(5), 720–8 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A and Keshavarzian A: Key role of PLC-gamma in EGF protection of epithelial barrier against iNOS upregulation and F-actin nitration and disassembly. Am J Physiol Cell Physiol, 285(4), C977–93 (2003) [DOI] [PubMed] [Google Scholar]
- 158.Dalle-Donne I, Giustarini D, Colombo R, Rossi R and Milzani A: Protein carbonylation in human diseases. Trends Mol Med, 9(4), 169–76 (2003) [DOI] [PubMed] [Google Scholar]
- 159.Aldini G, Dalle-Donne I, Facino RM, Milzani A and Carini M: Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev, 27(6), 817–68 (2007) [DOI] [PubMed] [Google Scholar]
- 160.Dalle-Donne I, Rossi R, Giustarini D, Gagliano N, Lusini L, Milzani A, Di Simplicio P and Colombo R: Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic Biol Med, 31(9), 1075–83 (2001) [DOI] [PubMed] [Google Scholar]
- 161.Cepinskas G, Kvietys PR and Aw TY: Omega 3-lipid peroxides injure CaCo-2 cells: relationship to the development of reduced glutathione antioxidant systems. Gastroenterology, 107(1), 80–6 (1994) [DOI] [PubMed] [Google Scholar]
- 162.Hecht D and Zick Y: Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun, 188(2), 773–9 (1992) [DOI] [PubMed] [Google Scholar]
- 163.Zhang ZY: Protein-tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit Rev Biochem Mol Biol, 33(1), 1–52 (1998) [DOI] [PubMed] [Google Scholar]
- 164.Chen YH and Lu Q: Association of nonreceptor tyrosine kinase c-yes with tight junction protein occludin by coimmunoprecipitation assay. Methods Mol Biol, 218, 127–32 (2003) [DOI] [PubMed] [Google Scholar]
- 165.Kevil CG, Okayama N and Alexander JS: H(2)O(2)-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol, 281(6), C1940–7 (2001) [DOI] [PubMed] [Google Scholar]
- 166.Kale G, Naren AP, Sheth P and Rao RK: Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun, 302(2), 324–9 (2003) [DOI] [PubMed] [Google Scholar]
- 167.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A and Rao R: Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J, 402(2), 291–300 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y and Tsukita S: Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol, 137(6), 1393–401 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wong V: Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol, 273(6 Pt 1), C1859–67 (1997) [DOI] [PubMed] [Google Scholar]
- 170.Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, Yokoyama M and Staddon JM: Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem, 276(13), 10423–31 (2001) [DOI] [PubMed] [Google Scholar]
- 171.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T and Ohno S: Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol, 152(6), 1183–96 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Banan A, Zhang LJ, Farhadi A, Fields JZ, Shaikh M, Forsyth CB, Choudhary S and Keshavarzian A: Critical role of the atypical {lambda} isoform of protein kinase C (PKC-{lambda}) in oxidant-induced disruption of the microtubule cytoskeleton and barrier function of intestinal epithelium. J Pharmacol Exp Ther, 312(2), 458–71 (2005) [DOI] [PubMed] [Google Scholar]
- 173.Banan A, Fields JZ, Farhadi A, Talmage DA, Zhang L and Keshavarzian A: Activation of delta-isoform of protein kinase C is required for oxidant-induced disruption of both the microtubule cytoskeleton and permeability barrier of intestinal epithelia. J Pharmacol Exp Ther, 303(1), 17–28 (2002) [DOI] [PubMed] [Google Scholar]
- 174.Rochat T, Burkhard C, Finci-Cerkez V and Meda P: Oxidative stress causes a protein kinase C-independent increase of paracellular permeability in an in vitro epithelial model. Am J Respir Cell Mol Biol, 9(5), 496–504 (1993) [DOI] [PubMed] [Google Scholar]
- 175.Yuan SY: Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol, 39(4–5), 213–23 (2002) [DOI] [PubMed] [Google Scholar]
- 176.DeFouw LM and DeFouw DO: Differential phosphodiesterase activity contributes to restrictive endothelial barrier function during angiogenesis. Microvasc Res, 62(3), 263–70 (2001) [DOI] [PubMed] [Google Scholar]
- 177.Huang Q and Yuan Y: Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol, 273(5 Pt 2), H2442–51 (1997) [DOI] [PubMed] [Google Scholar]
- 178.Li D and Mrsny RJ: Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol, 148(4), 791–800 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen Y, Lu Q, Schneeberger EE and Goodenough DA: Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell, 11(3), 849–62 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Y, Zhang J, Yi XJ and Yu FS: Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res, 78(1), 125–36 (2004) [DOI] [PubMed] [Google Scholar]