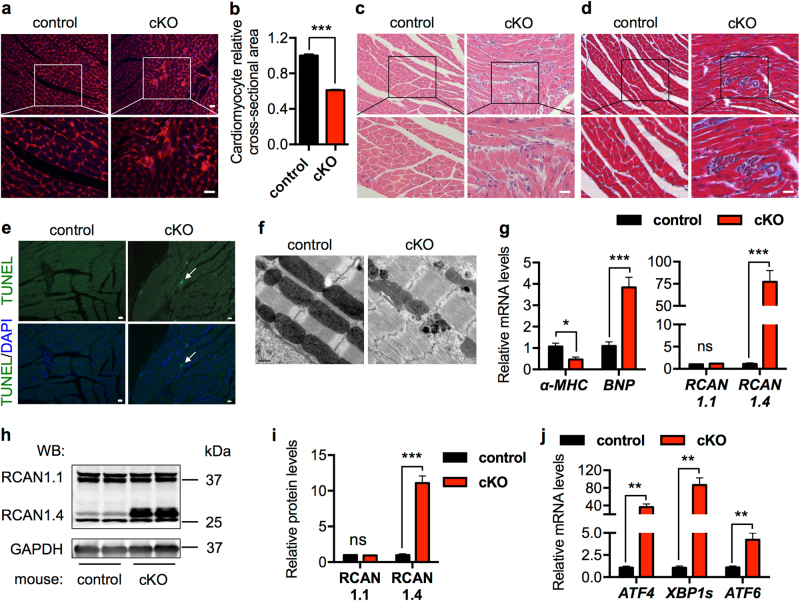
Fig. 5.
Cardiac-specific deficiency of GRP78 leads to more profound pathological cardiac remodeling. a Wheat germ agglutinin (WGA) staining supported that cardiac myocyte size was reduced in the cKO mice, which also showed clusters of non-cardiomyocyte infiltration. Blue indicates nuclei. Scale bar: 20 μm. b Quantification of WGA staining from a indicated a significant decrease in cardiomyocyte size in the GRP78 knockout myocardium. N = 100. c GRP78-deficient hearts displayed immune cell infiltration, which were clustered as patches and distributed in the myocardium. Scale bar: 20 μm. d Fibrosis was increased in the cKO heart compared to controls. Scale bar: 20 μm. e TUNEL staining revealed apoptotic cardiac cell death in the GRP78 cKO heart. Arrow indicates a TUNEL-positive cardiac myocyte. Scale bar: 20 μm. f Electron microscopic analysis of the hearts at day 14 post tamoxifen exposure. The cKO mice showed severe sarcomere disarray and mitochondrial disruption at the ultrastructure level. Scale bar: 0.5 μm. g GRP78 cKO caused pathological cardiac remodeling as shown by decreases in α-MHC and increases in BNP expression. Moreover, RCAN1.4, a marker of pathological remodeling, was significantly augmented, while the control RCAN1.1 showed no change. N = 6–8. h Protein level of RCAN1.4 was greatly stimulated in the GRP78 cKO mice. RCAN1.1 as a control did not differ. GAPDH was used as a loading control. i Quantification of h showed significant upregulation of RCAN1.4 in the absence of GRP78 in cardiac myocytes. N = 6. j GRP78 cKO in the heart led to strong induction of the UPR markers. N = 6–8. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant