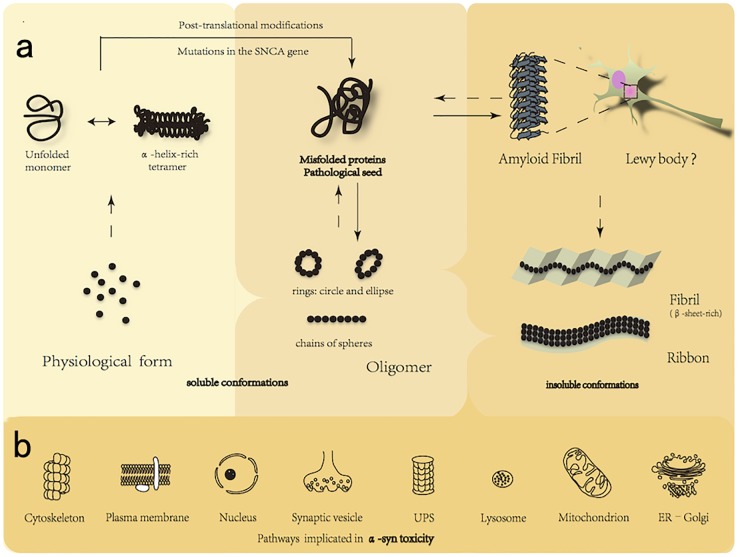
FIGURE 1.
(a) Native and toxic conformations of α-syn. Alpha-synuclein is able to transform into multiple different conformations, including monomers (predominant in a α-helical confirmation), tetramers, higher-level oligomers (soluble conformations), and fibrils (highly ordered insoluble conformations characterized by β-sheet conformation). Alpha-synuclein exists in a native conformation as monomers as well in a dynamic equilibrium with tetramers. The tetramer, less likely to form aggregate, must be first disrupted into monomer to further misfold. Toxic oligomers were also reported as being “on-pathway” or “off-pathway” to amyloid fibril formation. Many factors, such as the posttranscriptional modification and SNCA mutations in A53T and E46K promote to form pathological oligomers, presently considered to be the most toxic structure of α-syn, which is further folded to form amyloid fibril (rich in β-sheet structure), the accumulation of which leads to the formation of intracellular inclusions called Lewy Body. (b) Established interactions between α-syn and cellular components. The misfolded α-syn can be degraded by UPS and ALP. Certain oligomeric species present toxicity via interactions with cellular components by mechanisms that include: (1) alteration of cytoskeletal integrity; (2) membrane disruption and pore formation; (3) nuclear dysfunction; (4) inhibition of vesicle docking; (5) UPS dysfunction; (6) ALP impairment; (7) reduction of mitochondrial activity; and (8) chronic ER stress. UPS, ubiquitin-proteasomal system; ALP, autophagy-lysosomal pathway; ER, endoplasmic reticulum.