Abstract
Background
Intestinal fatty acid binding protein (I-FABP) has been shown to be a marker of intestinal damage among people living with HIV. We hypothesized that I-FABP would be increased in chronically HIV-infected patents more than elite controllers and would relate to specific nutrient intake and body composition.
Methods
In an observational study, serum I-FABP was measured by enzyme-linked immunosorbent assay. Anthropometric measurements, dual-energy x-ray absorptiometry, and single-slice abdominal computed tomography were obtained to assess body composition, as well as visceral and subcutaneous adipose tissue areas (VAT and SAT). Dietary intake was assessed using 4-day food records.
Results
One hundred forty-nine people with chronic HIV (65% male, 47 ± 7 years of age, 54.7% white, and 14 ± 6 years of known HIV), 10 elite controllers (60% male, 53 ± 8 years, 60% white, and 20 ± 7 years of known HIV), and 69 HIV-negative controls (59.4% male, 46 ± 7 years, and 52.2% white) were included in the analysis. I-FABP was significantly higher in HIV progressors relative to HIV-negative controls and elite controllers. In the chronic HIV group, I-FABP was positively associated with dietary intake of added sugar and with saturated fatty acids. I-FABP was inversely associated with body mass index, VAT, and SAT. I-FABP also correlated with MCP-1, CXCL10, sCD163, and lipopolysaccharide (LPS) among all participants.
Conclusions
I-FABP was increased among chronically HIV-infected patients to a greater degree than in elite controllers and was related to nutrient intake and body composition in HIV progressors. Future studies to investigate the role of intestinal damage on nutrient absorption are needed to elucidate the mechanisms of these relationships.
Trial Registration Identifier
Keywords: body composition, HIV, intestinal fatty acid binding protein, microbial translocation, nutrition
The gastrointestinal mucosa in people living with HIV (PLWH) is abnormal, in part due to loss of mucosal Th17 cells and weakened epithelial integrity, which contribute to increased microbial translocation [1, 2]. Intestinal fatty acid binding protein (I-FABP) has been shown to be a marker of intestinal damage among PLWH [3–6], and higher circulating I-FABP is associated with increased mortality and lower CD4+ T-cell count [7–10]. I-FABP is a cytosolic protein found in enterocytes of the gastrointestinal tract, most prominently in the jejunum. In healthy individuals, it is primarily involved in the translocation of fatty acids from the apical membrane of enterocytes to the endoplasmic reticulum, where fatty acids are converted into triglycerides [11]. Higher levels of circulating I-FABP have been reported in disease conditions where the intestinal wall is injured or compromised, such as sepsis, colitis, obesity, heart failure, and HIV [7, 12–16].
Serum I-FABP has been associated with activation of the innate immune system and contributes to chronic immune activation and systemic inflammation in the HIV population [5, 17, 18]. These alterations in the gastrointestinal tract may play a role in metabolic dysregulation. In this study, for the first time, we compared I-FABP concentrations in chronically infected patients, elite controllers, and non-HIV controls. We also assessed the relationship between serum I-FABP and sugar intake, which is known to affect gut barrier function, as well as other detailed measures of nutrient intake and body composition indices. We hypothesized that serum I-FABP would be increased in chronically HIV-infected patients more than elite controllers and would relate to indices of nutrient intake.
METHODS
Study Design
We assessed I-FABP in 150 individuals with chronic HIV and 69 HIV-negative controls who participated in an observational study at Massachusetts General Hospital. Participants with chronic HIV and HIV-negative controls were recruited from communities in the Boston area. Efforts were made to recruit participants with HIV and HIV-negative controls from similar communities. Many of the HIV-negative controls were friends, partners, or family members of the chronic HIV individuals. HIV-negative controls were confirmed to be HIV-negative by chemiluminometric immunoassay and confirmed by Western blot if positive. Controls of similar age, gender, and body mass index (BMI) were prospectively enrolled in the study at the same time as the HIV participants. The elite controllers were recruited from the Ragon Institute International HIV Controllers Study [19, 20] and underwent the same study procedures. Elite controllers were defined as persons who are antiretroviral therapy (ART)–naïve with undetectable viral load and without “viral blips.” They were included for comparison of I-FABP levels between groups. However, as elite controllers differ immunologically from chronic HIV, further analyses of relationships with I-FABP were undertaken without including the elite controllers. Exclusion criteria included known cardiac disease or symptoms consistent with angina. Participants with contraindication to beta blockers and nitroglycerin were also excluded. Details of the cohort have previously been published [21, 22]. All participants provided informed consent before enrollment. This study was approved by the institutional review boards of Massachusetts General Hospital and Massachusetts Institute of Technology.
Body Composition and Dietary Assessment
Height (cm) was measured (without shoes) in triplicate using a wall-mounted stadiometer (Holtain, Ltd.), and weight (kg) was measured (in hospital gown, without shoes) using a calibrated digital scale (Tanita Corporation of America Inc.) using standardized techniques in the fasting state. To assess visceral and subcutaneous adipose tissue areas (VAT and SAT, respectively), a cross-sectional abdominal computed tomography scan at the level of the L4 pedicle was performed [23]. Dual-energy x-ray absorptiometry (DXA, Hologic Horizon A, APEX software, version 5.6.0.5; Hologic Inc., Waltham, MA) was used to determine total body fat mass, total lean mass, and total body mass. Four-day food records (3 weekdays and 1 weekend day) were completed by the participants and reviewed for completeness by research dietitians. Food record data were analyzed using Nutrition Data System for Research (NDSR) software, version 2014, developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, Minnesota.
Inflammatory and HIV-Related Parameters
All participants were asked to fast overnight for 12 hours before the blood draw. Serum was obtained from fresh blood in real time, then aliquoted and stored in –80ºF freezers. Using these frozen sera, I-FABP was measured in duplicate using enzyme-linked immunosorbent assay (ELISA; R and D) in the laboratory of Dr. Tricia Burdo at Temple University. All measurements were performed in duplicate, with variation between duplicates <10%, positive controls, and standard curve. MCP-1, CXCL10, IL-6, and sCD14 were measured by ELISA (R and D), and sCD163 was also measured by ELISA (Trillium) under the same fasting conditions as I-FABP. In participants who were acutely ill, blood draw was delayed until they had fully recovered. CD4+ T-cell counts were assessed by flow cytometry. HIV viral load was determined by ultrasensitive real-time polymerase chain reaction. The limit of detection for our assay changed during the study from 50 to 48 copies/mL. Thus, those with an undetectable viral load were inputted as having either 49 or 47 copies/mL. HIV testing was performed in HIV-negative controls by chemiluminometric immunoassay and confirmed by Western blot.
Statistical Analysis
Comparisons between chronic HIV, elite controllers, and HIV-negative control groups were performed using analysis of variance or the Kruskal-Wallis test depending on the normality of distribution. For 2 group comparisons, the Student t test was performed for normally distributed continuous variables, and results were reported as mean ± standard deviation. The Wilcoxon rank-sum test was performed if the distribution was non-normal, and results were reported as median (interquartile range). The Spearman correlation coefficient was used to assess correlations with I-FABP, as I-FABP had a non-normal distribution. Linear regression analysis was used for adjusted analyses for continuous outcome variables. One outlier in the chronic HIV group with an extremely high I-FABP level (32 times the standard deviation) was excluded. Additional sensitivity analyses including the outlier showed that our primary results remained unchanged. A sensitivity analysis was also performed among participants with chronic HIV and suppressed HIV RNA, and the primary results remained unchanged. P < .05 was considered statistically significant. All statistical analyses were performed using SAS JMP.
RESULTS
Characteristics of the Participants
Baseline characteristics including age, gender, BMI, diabetes, tobacco use, low-density lipoprotein, and total cholesterol were similar between the chronic HIV group and the non-HIV control group (Table 1). Ten HIV elite controllers (who were not on ART and who had undetectable HIV RNA) were included for comparison of serum I-FABP to chronic HIV and HIV-negative controls. Elite controllers (53 ± 8 years) were slightly older than the chronic HIV participants (47 ± 7 years). Elite controllers also had a longer duration of known HIV diagnosis compared with chronic HIV participants (20 ± 7 years vs 14 ± 6 years, respectively; P = .03). Fifty percent of chronic HIV participants were on protease inhibitors, 35.4% were on non-nucleoside reverse transcriptase inhibitors, and only 12.4% were taking integrase inhibitors. Total duration of ART use was 7 ± 5 years. In the chronic HIV group, 99% of participants were on ART. The average CD4 counts in the chronic HIV group and the elite controllers were 552.8 ± 290 and 1010 ± 469 cells, respectively. Eighty-four percent of chronic HIV participants had an undetectable HIV RNA; therefore, an additional sensitivity analysis was carried out among only chronic HIV progressors with undetectable HIV RNA in the comparison of I-FABP between groups.
Table 1. .
Baseline Characteristics of Individuals With Chronic HIV, Elite Controllers, and HIV-Negative Controls
| Chronic HIV (n = 149) |
Elite Controllers (n = 10) |
HIV-Negative (n = 69) |
P Value HIV vs Elite Controllers |
P Value HIV vs HIV-Negative |
ANOVA/Kruskal-Wallis P Value Among 3 Groups |
|
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | 47 ± 7 | 53.4 ± 8 | 46 ± 7 | .03 | .21 | .03 |
| Male gender | 97 (65.1) | 6 (60) | 41 (59.4) | .74 | .42 | .71 |
| Race | .40 | .43 | .27 | |||
| Asian | 1 (0.7) | 0 | 3 (4.4) | |||
| White | 81 (54.7) | 6 (60.0) | 36 (52.2) | |||
| Black/African American | 56 (37.8) | 2 (20.0) | 25 (36.2) | |||
| American Indian | 1 (0.7) | 0 | 1 (1.5) | |||
| More than 1 race | 7 (4.7) | 1 (10) | 4 (5.8) | |||
| Hispanic | 14 (9.4) | 1 (10) | 4 (5.9) | .95 | .38 | .67 |
| Active smoker, % | 42.0 | 40.0 | 37.7 | .91 | .56 | .84 |
| Systolic BP, mmHg | 119 ± 14 | 120 ± 13 | 117 ± 14 | .74 | .36 | .57 |
| Diastolic BP, mmHg | 76 ± 9 | 76 ± 10 | 76 ± 9 | .99 | .97 | .99 |
| HIV-related parameters | ||||||
| Duration of known HIV, y | 14 ± 6 | 20 ± 7 | .03 | |||
| CD4+ cells, cells/m3 | 548 ± 289 | 1010 ± 469 | .01 | |||
| Viral load, copies/mLa | 49 [47–49] | Below detection limit by definition | ||||
| Suppressed viral load, % | 84 | 100 | .18 | |||
| Currently on ART, % | 99.3 | 0 | <.0001 | |||
| Duration of ART, y | 7 ± 5 | |||||
| NNRTI, % | 35.4 | |||||
| Protease inhibitor, % | 50.0 | |||||
| Integrase inhibitor, % | 12.4 | |||||
| Metabolic parameters | ||||||
| BMI, kg/m2 | 26.8 ± 5.1 | 28.3 ± 5.3 | 27.7 ± 4.8 | .38 | .20 | .33 |
| VAT, cm2 | 140 ± 107 | 124 ± 96 | 127 ± 100 | .62 | .40 | .66 |
| SAT, cm2 | 218 ± 135 | 278 ± 163 | 269 ± 154 | .29 | .02 | .03 |
| Hemoglobin A1c, % | 5.5 ± 0.8 | 6.0 ± 0.8 | 5.6 ± 0.5 | .11 | .11 | .07 |
| Total cholesterol, mg/dL | 182 ± 40 | 184 ± 53 | 181 ± 35 | .89 | .81 | .95 |
| LDL, mg/dL | 103 ± 33 | 107 ± 47 | 107 ± 31 | .76 | .32 | .62 |
| HDL, mg/dL | 52 ± 18 | 56 ± 18 | 53 ± 15 | .60 | .90 | .86 |
| Triglycerides, mg/dL | 134 ± 93 | 107 ± 68 | 103 ± 62 | .26 | .004 | .03 |
| Statin use, % | 13 | 10 | 4.5 | .78 | .06 | .20 |
| Marker of intestinal mucosal damage | ||||||
| I-FABP, pg/mL | 3458 [2023–4686] | 1947 [1612–2650] | 1633 [1149–2127] | .03 | <.0001 | <.0001 |
Data are reported as mean ± standard deviation, median [interquartile range], No. (%), or percentage.
Abbreviations: ANOVA, analysis of variance; ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; I-FABP, intestinal fatty acid protein; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; Y, years.
aThe limit of detection for our assay changed during the study from 50 to 48 copies/mL. Thus, those with undetectable viral load were inputted as having either 49 or 47 copies/mL.
I-FABP Among the Participants
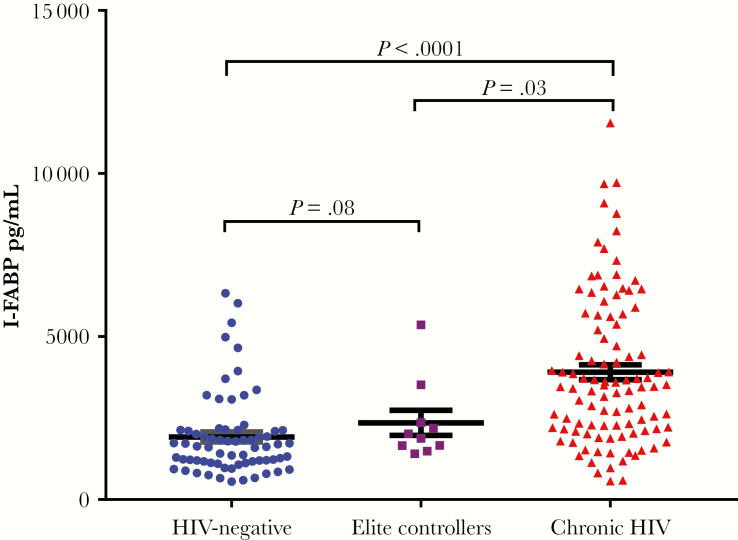
Serum I-FABP was significantly higher in participants with chronic HIV (3458 [2023–4686] pg/mL) compared with HIV-negative controls (1633 [1149–2127] pg/mL; P < .0001) and elite controllers (1947 [1612–2650] pg/mL; P = .03) (Figure 1). In a sensitivity analysis, serum I-FABP remained significantly elevated in participants with chronic HIV with undetectable HIV RNA (3506 [2114–4940] pg/mL) when compared with HIV-negative controls (P < .0001) and elite controllers (P = .03). Although there were small differences in age and duration of known HIV infection between the chronic HIV and elite controllers in our cohort, I-FABP did not correlate with age (ρ = .05, P = .42) or with duration of known HIV diagnosis (ρ = –.03, P = .66). Age and duration of known HIV infection are thus less likely to be confounders. Stratifying by gender, I-FABP was significantly higher in women with chronic HIV compared with HIV-negative women (3460 [2040–5015] pg/mL vs 1576 [1125–2254] pg/mL, respectively; P < .0001). Men with chronic HIV had significantly elevated I-FABP compared with HIV-negative men (3118 [1887–4527] pg/mL vs 1633 [1187–1975] pg/mL, respectively; P < .0001).
Figure 1.
Blue circles denote HIV-negative controls, purple squares denote elite controllers, and red triangles are chronic HIV. Abbreviation: I-FABP, intestinal fatty acid protein.
Association of Serum I-FABP and Dietary Intake
There was no difference in total caloric, fat, carbohydrate, protein, total saturated fatty acid (SFA), total monounsaturated fatty acid (MUFA), and total polyunsaturated fatty acid (PUFA) intake between HIV-negative controls and HIV-infected participants. Of note, serum I-FABP significantly correlated with intake of SFA 4:0 (ρ = .21, P = .02), SFA 6:0 (ρ = .21, P = .02), SFA 8:0 (ρ = .24, P = .008), SFA 10:0 (ρ = .19, P = .03), SFA 12:0 (ρ = .20, P = .03), SFA 17:0 (ρ = .19, P = .04), SFA 20:0 (ρ = .21, P = .02), and SFA 22:0 (ρ = .20, P = .02) in the chronic HIV group. There was no correlation between serum I-FABP and SFA intake among HIV-negative controls (Table 2B).
Table 2A.
Comparison of Nutritional Intake Between HIV-Negative Controls and Individuals With HIV
| Nutritional Intake | Chronic HIV Patients, Median [IQR] | HIV-Negative Controls, Median [IQR] | P Value |
|---|---|---|---|
| Total energy, kcal | 2077 [1576–2793] | 1946 [1334–2570] | .28 |
| Total fat, g | 81 [60–109] | 78 [47–111] | .39 |
| Total carbohydrate, g | 247 [181–340] | 219 [165–296] | .05 |
| Total protein, g | 86 [66–114] | 89 [62–118] | .90 |
| Total cholesterol, mg | 288 [192–400] | 280 [188–384] | .48 |
| Total SFA, g | 28 [20–36] | 26 [16–35] | .19 |
| Total MUFA, g | 31 [22–40] | 29 [19–42] | .60 |
| Total PUFA, g | 16 [10–22] | 15 [10–21] | .70 |
| Total sugar, g | 104 [73–156] | 77 [46–112] | .007 |
| Added sugar, g | 63 [38–107] | 45 [30–69] | .008 |
| Sucrose, g | 38 [26–66] | 31 [18–41] | .006 |
| Fructose, g | 22 [12–33] | 15 [8–28] | .03 |
Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Total sugar intake was significantly higher in the HIV-infected group than in the HIV control group (104 [73–156] g vs 77 [46–112] g, respectively; P = .007). The HIV-infected group also had higher intake of added sugar (63 [38–107] g vs 45 [30–70] g, respectively; P = .008) and sucrose (38 [26–66] g vs 31 [18–41] g, respectively; P = .006) compared with HIV-negative controlsB (Table 2A). Interestingly, I-FABP directly correlated with intake of added sugar (ρ = .22, P = .03) and sucrose (ρ = .27, P = .003) in the HIV-infected group (Table 2B). However, this was not observed in HIV-negative controls.
Table 2B.
Relationships Between Serum I-FABP and Nutritional Factors Among Individuals With HIV
| Spearman ρ | P Value | |
|---|---|---|
| Total energy, kcal | .10 | .26 |
| Total fat, g | .12 | .17 |
| Total carbohydrate, g | .12 | .18 |
| Total protein, g | –.06 | .55 |
| Total cholesterol, mg | .005 | .95 |
| Total SFA, g | .14 | .13 |
| SFA 4:0 | .21 | .02 |
| SFA 6:0 | .21 | .02 |
| SFA 8:0 | .24 | .008 |
| SFA 10:0 | .19 | .03 |
| SFA 12:0 | .20 | .03 |
| SFA 14:0 | .16 | .09 |
| SFA 17:0 | .19 | .04 |
| SFA 20:0 | .21 | .02 |
| SFA 22:0 | .20 | .02 |
| Total MUFA, g | .10 | .30 |
| Total PUFA, g | .20 | .03 |
| Total sugar, g | .16 | .07 |
| Added sugar, g | .22 | .03 |
| Sucrose, g | .27 | .003 |
Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Association of I-FABP Level With Body Composition, BMI, VAT, and SAT
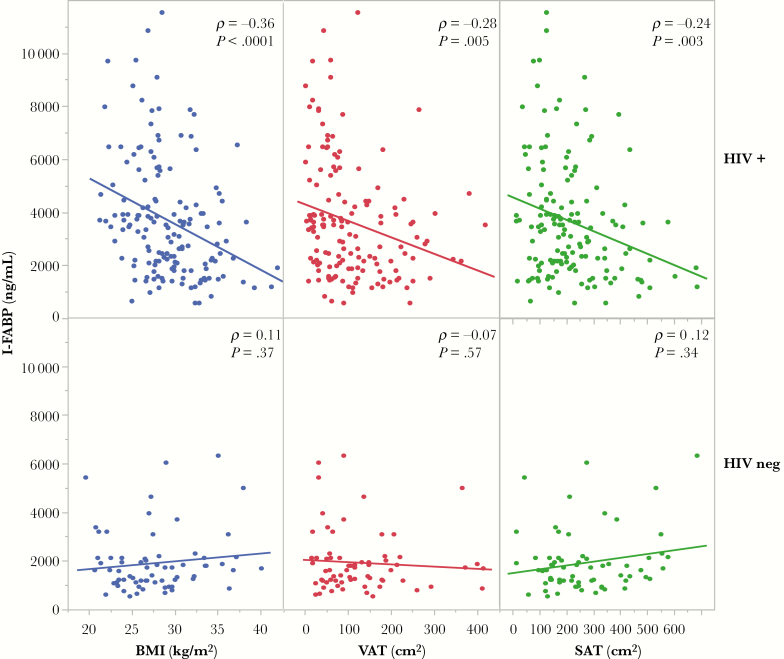
Higher levels of serum I-FABP were inversely associated with weight (ρ = –.33, P ≤ .0001), BMI (ρ = –.36, P ≤ .0001), SAT (ρ = –.24, P = .003), and VAT (ρ = –.28, P = .0005) in the HIV-infected group (Figure 2). Serum I-FABP also correlated negatively with waist circumference (ρ = –.35, P < .0001) and hip circumference (ρ = –.30, P = .0003) in the HIV-infected group (Table 3). These relationships were not observed in the HIV-negative control group. We analyzed LPS to further assess the inverse relationship observed between gut damage and body composition. Lipopolysaccharide (LPS) was inversely and significantly associated with lower total body fat (ρ = –.21, P = .02), percent body fat (ρ = –.30, P = .001), total lean body mass (ρ = –.30, P = .001), and SAT (ρ = –.23, P = .01). There was an inverse trend between LPS and BMI (ρ = –.14, P = .11).
Figure 2.
Blue circles denote body mass index, red circles denote visceral adipose tissue, and green circles denote subcutaneous adipose tissue. Abbreviations: BMI, body mass index; I-FABP, intestinal fatty acid binding protein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Table 3.
Relationships of Serum I-FABP With Measures of Body Composition in Individuals With Chronic HIV and HIV-Negative Controls
| Chronic HIV, Spearman ρ | P Value | HIV-Negative, Spearman ρ | P Value | |
|---|---|---|---|---|
| BMI | –.36 | <.0001 | .11 | .37 |
| VAT | –.28 | .0005 | –.07 | .57 |
| SAT | –.24 | .003 | .12 | .34 |
| Total body fat | –.26 | .001 | .08 | .50 |
| Total body fat | –.15 | .07 | .11 | .39 |
| Total lean body mass | –.21 | .008 | –.05 | .70 |
| Total mass | –.33 | <.0001 | .06 | .64 |
Abbreviations: BMI, body mass index; I-FABP, intestinal fatty acid binding protein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Relationship of Serum I-FABP With Inflammatory Markers
Serum I-FABP was positively related to MCP-1 (ρ = .20, P = .005), CXCL10 (ρ = .30, P = .008), sCD163 (ρ = .26, P ≤ .0001), and LPS (ρ = .16, P = .03) among all participants. There was a trend toward significance in the relationship between I-FABP and sCD14 (ρ = .12, P = .07). I-FABP did not correlate with IL-6 (ρ = –.07, P = .37).
Multivariate Analyses
In a multivariate model adjusting for markers of inflammation significantly associated with I-FABP (MCP-1, CXCL10, and sCD163), CD4+ cell count, and HIV viral load, the relationship between I-FABP and BMI (β = –.0008, P = .02), VAT (β = –.01, P = .01), and SAT (β = –.03, P = .001) remained significant. In contrast, the relationship between I-FABP and lean body mass was no longer significant after adjusting for these inflammatory and HIV-related parameters (β = –.0003, P = .43). In further analysis adjusting for age, race, sex, duration of known HIV, duration of ART use, and viral load, the relationship between I-FABP and BMI (β = –.001, P = .01), VAT (β = –.011, P = .007), and SAT (β = –.017, P = .02) remained significant.
DISCUSSION
In this study, for the first time, we report higher serum I-FABP in people with chronic HIV relative to elite controllers. Elite controllers also tended to have higher I-FABP than HIV-negative controls. To our knowledge, this is the first published report of serum I-FABP level in elite controllers. We also demonstrated that dietary intake of saturated fatty acids and added sugar positively correlate with higher serum I-FABP in people with chronic HIV. Additionally, we observed an inverse relationship between serum I-FABP and BMI, VAT, and SAT (and other measures of adiposity) in people with chronic HIV that has not been previously described. We also observed a positive relationship between serum I-FABP and markers of monocyte activation (sCD163, MCP-1, CXCL10) and LPS, consistent with prior studies in PLWH [11, 16, 17].
Prior studies have carefully characterized intestinal damage in HIV and established elevated levels of circulating I-FABP as a marker of intestinal damage in PLWH [5, 7, 24, 25]. Chronic intestinal inflammation and mucosal damage can have detrimental health consequences in PLWH [7]. Hunt et al. measured I-FABP in the SCOPE and LSOCA cohorts and found that I-FABP predicted mortality in people with ART-suppressed HIV infection with a history of AIDS. HIV-infected participants from the LSOCA cohort and our chronic HIV participants had comparable I-FABP levels. However, the SCOPE cohort, which had less advanced immunodeficiency than the LSOCA cohort, had lower I-FABP compared with our chronic HIV group [7]. There are differences in selection criteria in each cohort, making direct comparisons difficult. Our study included more women than the cohorts in Hunt et al. and included a focus on I-FABP’s relationship with body composition and dietary data that was not previously assessed in other studies in HIV. Additionally, our cohort included well-matched HIV-negative controls and is the first to describe I-FABP in elite controllers in comparison with chronic HIV patients and HIV-negative controls.
Our finding of lower circulating I-FABP among elite controllers compared with progressors suggests that there is less intestinal mucosal damage in elite controllers. These findings complement prior studies that have shown that elite controllers have preserved mucosal CD4+ T cells in the intestinal mucosa and a more robust immune response in intestinal mucosa [26, 27]. Thus, relative preservation of mucosal barrier function may be an important characteristic of HIV elite controllers that helps to prevent disease progression. However, a trend was observed for higher I-FABP in the elite controllers compared with HIV-negative controls, suggesting that there may still be some mild degree of intestinal damage in elite controllers.
I-FABP is a cytosolic protein expressed specifically in the mature enterocytes of the small intestine [28, 29]. Examining the biological function of I-FABP protein may offer additional insight into our findings. I-FABP has a high affinity for binding saturated and unsaturated long-chain fatty acids and has been presumed to play a role in lipid binding and trafficking within the enterocyte [11]. High-fat diets have been shown to increase I-FABP expression [30, 31]. These findings suggest a possible lipid-sensing role for I-FABP or a role of a high-fat diet in causing intestinal damage. In healthy individuals, basal I-FABP levels in plasma reflect normal enterocyte turnover, whereas higher levels may signal intestinal epithelial damage in certain disease conditions. The higher levels of I-FABP in people living with chronic HIV are likely indicative of intestinal epithelial damage [7].
One of the pathologic alterations in PLWH is destruction of the normal intestinal function and structure, including blunting of intestinal microvilli. Thus, individuals may develop malabsorption of micronutrients. Our finding of lower BMI, VAT, and SAT (and lower total lean and fat mass) in individuals with higher-serum I-FABP may therefore relate to malabsorption from chronic intestinal damage. The ensuing chronic inflammation related to chronic intestinal microbial translocation may also cause further loss of fat and muscle. Timmons et al. had previously reported an inverse correlation between sCD14, a marker of microbial translocation, and lean mass, trunk, and limb fat [32]. Our data further extend these findings by demonstrating an inverse relationship of LPS and I-FABP with muscle and adipose tissue, implicating the disruption of the gastrointestinal (GI) tract mucosa as a potential cause of muscle and fat loss. Their data, when combined with ours, suggest that intestinal damage and microbial translocation in PLWH can potentially cause loss of lean mass and fat. Although we saw an inverse relationship between I-FABP and total body lean mass, this relationship was no longer significant after adjusting for inflammatory and HIV-related factors, suggesting that loss of muscle mass may be more likely due to chronic inflammation. In contrast, the relationship between I-FABP and adipose tissue measures remained significant even after adjusting for inflammatory and HIV-related parameters, indicating an independent relationship between I-FABP and adiposity.
To rule out diet and caloric intake as potential contributors to differences observed in I-FABP levels between PLWH and HIV-negative controls, we reviewed nutritional intake assessed by 4-day food records. Total calories, total fat, total carbohydrate, total SFA, total MUFA, and total PUFA intake did not differ significantly between the HIV-negative controls and chronic HIV participants. PLWH had a diet consisting of higher total sugar and added sugar intake relative to HIV-negative individuals. Serum I-FABP positively correlated with added sugar intake and SFA intake in PLWH. The relationships between I-FABP and added sugar intake or with saturated fat have not been previously described in PLWH. We hypothesize that PLWH who consume excess sugar and/or a high-fat diet likely have more I-FABP released by enterocytes due to further diet-related intestinal damage in the setting of an already compromised intestinal epithelium. Indeed, recent key scientific studies have shown that local high-glucose concentrations can be a driver of intestinal epithelial damage [33] and that saturated fat [34] can induce damage to the intestinal mucosa. In another model of abnormal intestinal inflammation, Lee et al. previously demonstrated similar results in a mouse model of inflammatory bowel disease (IBD), in which IBD mice fed a high-fat diet developed changes in the gut microbiota, intestinal damage, and increased susceptibility to colitis compared with IBD mice on a standard diet [35]. Another rodent study evaluating the effect of a diet high in SFA showed that rats who consumed a diet high in SFA failed to gain weight and developed lower jejunal and ileal surface area [36]. These animal studies may help to elucidate the impact of diet on intestinal damage in our HIV cohort.
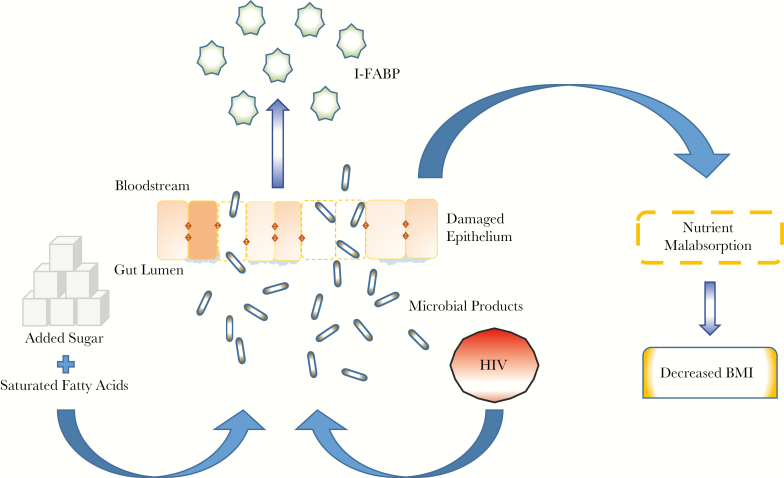
Although the exact mechanism is unclear, our findings highlight the important role of diet (and especially added sugar and saturated fatty acids) on intestinal function and body composition in PLWH. Taken together, these data suggest a potential schema, whereby excess sugar and SFA intake may contribute to further gut damage in people with HIV, leading to impaired absorption and lower BMI (Figure 3). Alternatively, in PLWH, low BMI may be associated with intestinal damage leading to compensatory increases in dietary sugar and fat intake. Although the directionality is unknown, these data are the first to suggest the need for specific dietary interventions to see if reducing sugar and saturated fat intake will ameliorate intestinal mucosal damage in chronically HIV-infected patients and further elucidate the mechanisms of this relationship.
Figure 3.
Proposed mechanisms of intestinal damage, intestinal fatty acid binding protein release, and effects on body composition. Abbreviations: BMI, body mass index; I-FABP, intestinal fatty acid binding protein.
An alternative hypothesis for the observed relationship of I-FABP and adiposity in PLWH is the potential role of the gastrointestinal microbiome in mediating this relationship. Extreme states of weight such as malnutrition and obesity affect the intestinal microbiota, which in turn can affect gastrointestinal permeability [37, 38]. Gastrointestinal microbiome alterations in PLWH are similar to microbiome changes observed in malnutrition, which include an increase in Proteobacteria and a decrease in some beneficial species within the Bacteroidetes and Firmicutes phyla [39, 40]. Bacteroides fragilis has been shown to have a protective effect on the intestinal barrier via expression of polysaccharide-A [41]. Conversely, Enterobacteriaceae of the Proteobacteria phylum is increased in PLWH and has been associated with immune activation and bacterial translocation [39, 42]. Of interest, Roseburia intestinalis of the Firmicutes phylum (a beneficial species in healthy flora) has been shown to be reduced in PLWH and in malnutrition [40, 43]. The decreased abundance of Roseburia intestinalis is also related to markers of microbial translocation and immune activation [43]. Therefore, intestinal dysbiosis can be a contributor to intestinal mucosal damage and therefore a potential driver of the rise in I-FABP observed in PLWH.
Our study has limitations. The cross-sectional design does not allow us to determine causal relationships, and we did not assess GI epithelial damage directly via biopsy. However, serum I-FABP level has been previously established as a marker of intestinal enterocyte damage. Our novel findings require further research to understand the mechanisms behind the observed inverse relationship between I-FABP and reduced fat in all depots in PLWH.
In conclusion, I-FABP is higher in people with chronic HIV compared with elite controllers and non-HIV controls. Dietary intake of SFA and added sugar is associated with elevated serum I-FABP among people with chronic HIV. Our findings suggest that dietary modification to reduce saturated fat and added sugar intake may help reduce further intestinal damage in PLWH. In addition, I-FABP is inversely related to BMI, VAT, and SAT in chronic HIV. Further research is warranted to elucidate the mechanisms by which circulating levels of I-FABP/enterocyte damage lead to alterations in BMI and body composition in PLWH. Additionally, it will be important to understand how nutritional factors such as saturated fatty acids and added sugar may affect intestinal health in PLWH. Future studies are needed in PLWH to identify treatment strategies to improve intestinal epithelial integrity. In addition to strategies currently being tested, such as probiotics and prebiotics [44–47], or glucagon-like peptide-2 to restore the epithelial barrier function, dietary modification to reduce added sugar intake and saturated fatty acids may be a potential future strategy to help improve intestinal health and mucosal barrier function in PLWH.
Acknowledgments
We wish to thank the participants of this study and the Nursing and Bionutrition Staff of the MGH and MIT Clinical Research Centers.
Author contributions. Study conception (J.L.); study design (J.L.); participant recruitment, history-taking, and physical examination (J.L., K.F., S.L.); data acquisition (J.L., K.V.F., T.B., J.R., J.H., M.T.); statistical analysis and interpretation (L.C., E.P., C.S., J.W., J.L.); drafting of the manuscript (L.C., E.P., J.L.); critical revision of manuscript (L.C., E.P., C.S., K.F., S.L., J.W., T.B., J.H., M.T., J.L.); and supervision of study (J.L.).
Financial support. This work was supported by the National Heart, Lung, and Blood Institute RO1HL123351 (J.L) and K23HL092792 (J.L.), the National Institute of Diabetes and Digestive and Kidney Diseases P30DK04561 (M.T.) and 5T32DK007028 (L.C.), the Harvard Clinical and Translational Science Center and the National Center for Research Resources funding number 1-UL1RR025758-04, and by Bristol-Myers Squibb. Funding sources had no direct role in the design of the study, data analysis, or writing of the manuscript.
Potential conflicts of interest. L.C., E.P., C.S., T.B., K.F., S.L., J.W., J.R., J.H., and M.T. have nothing to declare. J.L. participated in a Medical Affairs Advisory Board meeting for Gilead Sciences and served as a consultant for Viiv Healthcare. All declarations of interest of the co-authors are unrelated to the design of this study and the preparation of this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentations. Elements of this manuscript have been presented at CROI and ENDO 2018.
References
- 1. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013; 21:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther 2016; 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nazli A, Chan O, Dobson-Belaire WN, et al. . Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estes JD, Harris LD, Klatt NR, et al. . Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010; 6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 6. Jiang W, Lederman MM, Hunt P, et al. . Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michelini Z, Baroncelli S, Fantauzzi A, et al. . Reduced plasma levels of sCD14 and I-FABP in HIV-infected patients with mesalazine-treated ulcerative colitis. HIV Clin Trials 2016; 17:49–54. [DOI] [PubMed] [Google Scholar]
- 9. Perkins MR, Bartha I, Timmer JK, et al. ; Swiss HIV Cohort Study The interplay between host genetic variation, viral replication, and microbial translocation in untreated HIV-infected individuals. J Infect Dis 2015; 212:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunt PW, Martin JN, Sinclair E, et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 11. Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids 2015; 93:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox AJ, Zhang P, Bowden DW, et al. . Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab 2017; 43:163–6. [DOI] [PubMed] [Google Scholar]
- 13. Voth M, Duchene M, Auner B, et al. . I-FABP is a novel marker for the detection of intestinal injury in severely injured trauma patients. World J Surg 2017; 41:3120–7. [DOI] [PubMed] [Google Scholar]
- 14. Kitai T, Kim YH, Kiefer K, et al. . Circulating intestinal fatty acid-binding protein (I-FABP) levels in acute decompensated heart failure. Clin Biochem 2017; 50:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oldenburger IB, Wolters VM, Kardol-Hoefnagel T, et al. . Serum intestinal fatty acid-binding protein in the noninvasive diagnosis of celiac disease. APMIS 2018; 126:186–90. [DOI] [PubMed] [Google Scholar]
- 16. Derikx JP, Poeze M, van Bijnen AA, et al. . Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock 2007; 28:544–8. [DOI] [PubMed] [Google Scholar]
- 17. Ancuta P, Kamat A, Kunstman KJ, et al. . Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassol E, Malfeld S, Mahasha P, et al. . Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–33. [DOI] [PubMed] [Google Scholar]
- 19. Pereyra F, Lo J, Triant VA, et al. . Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pereyra F, Addo MM, Kaufmann DE, et al. . Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008; 197:563–71. [DOI] [PubMed] [Google Scholar]
- 21. Lo J, Abbara S, Shturman L, et al. . Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010; 24:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitch KV, Srinivasa S, Abbara S, et al. . Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borkan GA, Gerzof SG, Robbins AH, et al. . Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982; 36:172–7. [DOI] [PubMed] [Google Scholar]
- 24. Dinh DM, Volpe GE, Duffalo C, et al. . Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 2015; 211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Funderburg NT, Boucher M, Sattar A, et al. . Rosuvastatin decreases intestinal fatty acid binding protein (I-FABP), but does not alter zonulin or lipopolysaccharide binding protein (LBP) levels, in HIV-infected subjects on antiretroviral therapy. Pathog Immun 2016; 1:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferre AL, Hunt PW, Critchfield JW, et al. . Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 2009; 113:3978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guadalupe M, Reay E, Sankaran S, et al. . Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Auinger A, Helwig U, Rubin D, et al. . Human intestinal fatty acid binding protein 2 expression is associated with fat intake and polymorphisms. J Nutr 2010; 140:1411–7. [DOI] [PubMed] [Google Scholar]
- 29. Pelsers MM, Namiot Z, Kisielewski W, et al. . Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36:529–35. [DOI] [PubMed] [Google Scholar]
- 30. Lau E, Marques C, Pestana D, et al. . The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr Metab (Lond) 2016; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drozdowski L, Clement L, Keelan M, et al. . Dietary lipids modify intestinal lipid-binding protein RNA abundance in diabetic and control rats. Digestion 2004; 70:192–8. [DOI] [PubMed] [Google Scholar]
- 32. Timmons T, Shen C, Aldrovandi G, et al. . Microbial translocation and metabolic and body composition measures in treated and untreated HIV infection. AIDS Res Hum Retroviruses 2014; 30:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thaiss CA, Levy M, Grosheva I, et al. . Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018; 359:1376–83. [DOI] [PubMed] [Google Scholar]
- 34. Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. . Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015; 22:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee JC, Lee HY, Kim TK, et al. . Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS One 2017; 12:e0187515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drozdowski L, Woudstra T, Wild G, et al. . Dietary lipids modify the age-associated changes in intestinal uptake of fructose in rats. Am J Physiol Gastrointest Liver Physiol 2005; 288:G125–34. [DOI] [PubMed] [Google Scholar]
- 37. Blanton LV, Charbonneau MR, Salih T, et al. . Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016; 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ley RE, Bäckhed F, Turnbaugh P, et al. . Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinh DM, Volpe GE, Duffalo C, et al. . Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 2015; 211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tidjani Alou M, Million M, Traore SI, et al. . Gut bacteria missing in severe acute malnutrition, can we identify potential probiotics by culturomics?Front Microbiol 2017; 8:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Telesford KM, Yan W, Ochoa-Reparaz J, et al. . A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes 2015; 6:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marchetti G, Bellistrì GM, Borghi E, et al. . Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008; 22:2035–8. [DOI] [PubMed] [Google Scholar]
- 43. Dillon SM, Kibbie J, Lee EJ, et al. . Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017; 31:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kazemi A, Djafarian K, Speakman JR, et al. . Effect of probiotic supplementation on CD4 cell count in HIV-infected patients: a systematic review and meta-analysis. J Diet Suppl 2018; 15:776–88. [DOI] [PubMed] [Google Scholar]
- 45. González-Hernández LA, Jave-Suarez LF, Fafutis-Morris M, et al. . Synbiotic therapy decreases microbial translocation and inflammation and improves immunological status in HIV-infected patients: a double-blind randomized controlled pilot trial. Nutr J 2012; 11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirao LA, Grishina I, Bourry O, et al. . Early mucosal sensing of SIV infection by paneth cells induces IL-1β production and initiates gut epithelial disruption. PLoS Pathog 2014; 10:e1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klatt NR, Canary LA, Sun X, et al. . Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest 2013; 123:903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]