Abstract
Background aims:
CD1d-restricted invariant Natural Killer (iNK) T cells are rare regulatory T cells that may contribute to the immune-regulation in allogeneic stem cell transplantation (ASCT). Here, we sought to develop an effective strategy to expand human iNK T cells for use of cell therapy to prevent graft versus host disease (GVHD) in ASCT.
Methods:
Human iNK T cells were first enriched from peripheral blood mononuclear cells (PBMC) using magnetic-activated cell sorting separation, then co-cultured with dendritic cells in the presence of agonist glycolipids, alpha-galactosylceramide for 2 weeks.
Results:
The single antigenic stimulation reliably expanded iNK T cells to an average of 2.8×107 per 5×108 PBMC in an average purity of 98.8% in 2 weeks (N=24). The expanded iNK T cells contained a significantly higher level of CD4+ and central memory phenotype (CD45RACD62L+) compared to freshly isolated iNK T cells, and maintained their ability to produce both Th-1 (IFNγ and TNFα) and Th-2 type cytokines (IL-4, IL-5, and IL-13) upon antigenic stimulation or stimulation with Phorbol 12-myristate 13-acetate/ionomycin. Interestingly, expanded iNK T cells were highly autoreactive and produced a Th-2 polarized cytokine production profile after being co-cultured with dendritic cells alone without exogenous agonist glycolipid antigen. Lastly, expanded iNK T cells suppressed conventional T cell proliferation and ameliorated xenograft GVHD (Hazard Ratio 0.1266, p<0.0001).
Conclusion:
we have demonstrated a feasible approach for obtaining ex vivo expanded, highly enriched human iNK T cells for use in adoptive cell therapy to prevent GVHD in ASCT.
Keywords: Human iNK T cells, ex vivo expansion, cell therapy, GVHD
Introduction
Allogeneic hematopoietic stem cell transplantation (ASCT) remains the only curative immunotherapy for several hematologic malignancies through in part varying degrees of graft versus leukemia (GVL) effects[1, 2]. While relapse of the disease due to insufficient GVL effects is the leading cause for post-transplantation mortality, graft versus host disease (GVHD) is the most common post-transplantation complications occurring in approximately 50% of patients following ASCT. GVHD is often fatal without aggressive and timely treatment [3]. The mainstay of treatments for acute GVHD is corticosteroids and intensification of immunosuppressants. However, these treatments may delay the engraftment of stem cells, increase the risk of life threatening infections, and blunt GVL effects leading to the early relapse of leukemia[3]. Thus, novel strategies to maintain an optimal balance between GVHD and GVL by donor lymphocytes are needed to improve the clinical outcome of ASCT.
CD1d-restricted invariant Natural Killer (iNK) T cells are rare but powerful regulatory T cells that influence adaptive immune responses through their ability to produce a varying degree of both Th-1 and Th-2 type cytokines upon activation[4]. The iNK T cells are thought to play a role in preventing GVHD in ASCT [5–9]. For example, adoptive transfer of murine CD4+ iNK T cells has been shown to suppress acute and chronic GVHD through the expansion of conventional regulatory T cells [10–12], and activation of donor iNK T cells using Th-2 polarizing agonist glycolipid antigen or liposomal αGalCer can ameliorate GVHD in murine models [9, 13]. In addition, a handful of correlative preclinical studies demonstrated that the higher dose of CD4− iNK T cells in the allograft or early reconstitution of iNK T cells post ASCT is associated with lower incidence of acute GVHD[5, 14–16]. Unlike conventional regulatory T cells, iNK T cells may have additional graft versus leukemic effects through intrinsic NK-like properties or by promoting GVL by donor lymphocytes[17–19]. Therefore, iNK T cell based immunotherapy is a novel approach to potentially balance the GVHD and GVL effects of donor lymphocytes in ASCT. In this study, we explored a strategy to expand highly pure human iNK T cells from adult donors, and assessed their immunoregulatory function to prevent xenogenic GVHD in ASCT.
Materials and Methods
Materials
This study was performed in accordance with the research protocol approved by The University of Texas M.D. Anderson Institutional Review Committee. Informed written consent from all study subjects were waived as all leukoPaks from adult donors were purchased through the MDACC Blood Bank. T cell media (TCM) was used for cell culture, and contained RPMI 1640 supplemented with 10% fetal bovine serum, 55 μM 2-mercaptoethanol, 10 μg/ml gentamicin, 10 mM HEPES, and 1x non-essential amino acid and essential amino acid (Invitrogen, Carlsbad, CA). Anti-iNKT microbeads (6B11) were purchased from Miltenyi Biotech (San Diego, CA), and the following antibodies were purchased from BioLegend (San Diego, CA) or BD Bioscience (San Jose, CA): iNK TCR (6B11), CD4 (RPA-T4), CD8α (SK11), IFNγ (B27), TNFα (MAB11), IL-4 (8D4–8), IL-13 (JES10–5A2), CD3 (OKT3). The following cytokines used for cell culture were purchased from BioLegend (San Diego, CA) or PeproTech (Rocky Hill, NJ): IL-2, IL-4, GM-CSF, and IL-7. The agonist glycolipid, αGalCer was synthesized as previously described [20, 21].
Ex vivo expansion of iNK T cells
Monocyte-derived dendritic cells (DC) were generated as previously reported[22]. Briefly, peripheral blood mononuclear cells (PBMC) were prepared using Ficoll-Plaque density gradient centrifugation protocol. Monocytes were isolated via plastic adherence, and cultured in TCM containing IL-4 (100 ng/ml) and GM-CSF (200 IU/ml) for 5 days. After irradiation (5000 cGy), DC were cryopreserved until further use. Dendritic cells from a single donor were used to expand iNK T cells from up to 4 – 5 allogeneic donors. The iNK T cells were first enriched from 2×108 to 1×109 PBMC prepared from the entire leukopak using anti-iNKT-MicroBeads and Magnetic Activated Cell Sorting (MACS) separation according to the manufacturer’s instructions (Miltenyi Biotech). Subsequently, they wereco-cultured with 2×105 DC per well in 1–3 wells of 24 well tissue culture plate in TCM in the presence of αGalCer (100 nM) and IL-2 (200 IU/ml) for 10–14 days. Growth factor and TCM was replenished in every 2–3 days, but αGalCer was not.
Flow cytometry
Freshly isolated or expanded iNK T cells were subjected to multi-parameter flow cytometric analysis for the following surface markers: CD3, CD4, CD8α, iNK invariant TCRα chain (clone: 6B11), CD45RA, CD62L. Dead cells were excluded using Fixable Viable Stain 620 (BD Bioscience). For intracellular cytokine analysis, iNK T cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 30 ng/ml) and ionomycin (1 μg/ml) in the presence of monensin (1 μM) for 3 hours. After staining of surface antigens, cells were fixed and permeabilized using BD Cytofix//Cytoperm™ (BD bioscience), and stained for intracellular cytokines prior to multi-parameter flow cytometric analysis. An LSRFortessa™ Cell Analyzer (BD Bioscience, Franklin Lakes, NJ) was used to acquire samples and FlowJo version 10.3 software (Tree Star, Ashland, OR) was used for analysis.
The iNK T cell functional assay
Fifty thousand iNK T cells were stimulated by 25,000 DC pulsed with or without αGalCer (100 nM) in triplicates in a 96 well-plate for 48 hours, and culture supernatants were assessed for the presence of IL-4, IL-5, IL-13, IFNγ, TNFα, and GM-CSF using DuoSet® Elisa Development Systems (R&D systems, Minneapolis, MN) according to the manufacturer’s instruction. BD OptEIA™ (BD bioscience) was used as TMB substrate reagent, and Cytation 5 (BioTek, VT) was used to measure absorbance at 450 nm.
For human Th-1/Th-2/Th-17 cytokine array (Raybiotech, Norcross, GA), 2×105 iNK T cells and 1×105 irradiated DC were co-cultured with and without αGalCer (100 nM) in a 24 well plate for 48 hours. Culture supernatants were used for comprehensive Th-1, Th-2, and Th-17 cytokine analysis according to the manufacturer’s instruction.
In vitro suppression assay
The responder T cells from allogeneic PBMC were labeled with Carboxyfluoreschein succinimidyl ester (CFSE, Invitrogen, CA) at 1 μM for 30 min at 37°C according to manufacturer’s instruction. A total of 5×105 CFSE-labeled T cells were stimulated with anti-CD3 and CD28 antibodies (2 μg/ml) for 16 hours in a 96 well plate in quadruplicates, and unbound antibodies were thoroughly washed off. Subsequently, CFSE-labeled PBMC were co-incubated with 5×104 allogeneic control T cells or iNK T cells (suppressor) for 5 days, and subjected to flow cytometric analysis of remaining CFSE on responder cells. The percent proliferation was defined as a percentage of CFSElow responder/total CFSE+ responder.
Xenogeneic murine GVHD model.
All animal experiments were performed under the University of Texas Institutional Animal Care and Use Committee (IACUC)-approved protocols. NOD-scid IL2Rgammanull (NSG) mice (Jackson laboratory, Bar Harbor, ME) between age of 10 to 14 weeks were irradiated with 200 cGy using the 137Cs irradiator on day −1, and infused with 107 iNK T cell-depleted PBMC with or without 5×105 allogeneic iNK T cells (n=10, each group) or control T cells (n=5) on day 0. Subsequently, clinical signs of GVHD represented by weight loss were monitored daily, and moribund mice were euthanized per institutional guidelines. For the pathology evaluation for GVHD, xenografted NSG mice with or without iNK T cells (n=4, each group) were sacrificed on day 7. The liver, gut, skin, and lungs were harvested for a routine histopathology of formalin fixed tissue using hematoxylin and eosin staining. Analysis and scoring of sections for pathologic features of GVHD were carried out by veterinary pathologists. Briefly, the average number of individual necrotic cells per 40x objective was assessed for GI pathology, portal inflammation per 10x objective for liver pathology, and the area of lymphocyte infiltration per 4x objective for lung pathology. A minimum 200 views were assessed for GI pathology, 50 views for liver pathology, and all areas for lung pathology.
Statistical Analysis.
Analysis was performed using Prism version 6.0 (GraphPad, San Diego, CA). Data sets were analyzed using a paired student t-test with equal variance unless mentioned otherwise, and p values for comparisons between groups were determined. Differences in animal survival was estimated by log-rank test. A p value less than 0.05 was considered as “significant”
Results
A single antigenic stimulation led to rapid ex vivo expansion of highly pure human iNK T cells.
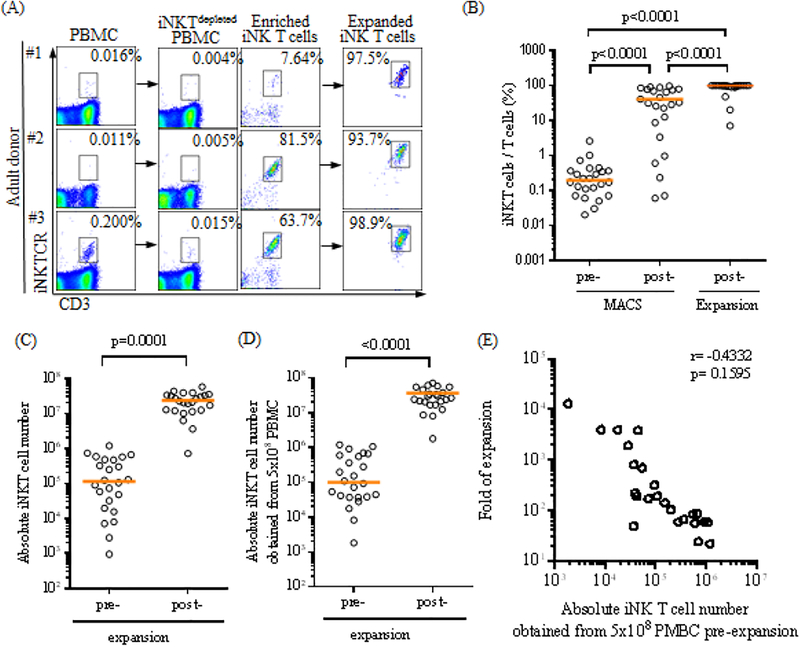
The iNK T cells are a rare population that constitutes about 0.1% of circulating T cells with a large donor to donor variation ranging from 0.001% up to ~3%[23]. In order to efficiently expand this extremely rare population, iNK T cells were first enriched from an entire leukoPak via MACS separation using commercially available anti-iNKT microbeads (clone 6B11), and then subjected to co-culture with monocyte-derived allogeneic dendritic cells in the presence of the agonist glycolipid, αGalCer and IL-2 for 10–14 days. As shown in Figure 1A and B, the purity of iNK T cells substantially increased after the first enrichment step, and consistently exceeded greater than 95 % after a single round of antigen specific expansion.
Figure 1.
A single antigenic stimulation efficiently expands highly pure human iNK T cells. (A-B) The iNK T cells were enriched from adult peripheral blood mononuclear cells (PBMC) via anti-iNKT microbeads/MACS separation, and subsequently co-cultured with allogeneic dendritic cells (DC) in the presence of αGalCer and IL-2 for 10–14 days. The representative flow cytometric analyses of iNK T cells pre and post MACS separation and post expansion from three donors were shown in (A), and percentages of iNK T cells from total T cells at each step from 24 consecutive donors were shown in (B). The purity of iNK T cells increased from average (median, hereafter) 0.195% pre-MACS separation to an average of 31.9% post MACS separation, and to 98.8% after a single round of antigen specific expansion (n=24). Absolute numbers of iNK T cells obtained before and after the expansion from 24 consecutive donors are shown in (C), and these values were adjusted to 5×108 PBMC (D). Folds of expansion were plotted against absolute numbers of iNK T cells obtained from 5×108 PMBC prior to the expansion (E). A total of 1.1×105 iNK T cells on average (range: 948 to 1.2×106) were obtained from 2×108 to 1×109 PBMC from 24 consecutive donors, and underwent an average of 156 folds of expansion (range 22 to 13186), resulting in an average of 2.1×107 iNK T cells (range: 7.2×105 to 5.7×107). When these values were adjusted to 5×108 PBMC, the average of 1.0×105 iNK T cells (range: 1823 to 1.2×106) were obtained from 5×108 PBMC, and an average of 2.8×107 iNK T cells (range: 1.8×106 to 1.7×108) were obtained after the expansion. There was a trend towards achieving the higher folds of expansion when the lower absolute number of iNK T cells were obtained prior to the expansion, but this was not statistically significant. A single symbol represents a value from a single donor. The median was used as the average value. A paired student t-test was used to compare the differences between selected groups. P-value less than 0.05 was considered as “significant”
In order to demonstrate the feasibility of developing iNK T cell-based adoptive cell therapy, we expanded polyclonal iNK T cells from 24 consecutive adult donors and assessed folds of expansion, absolute numbers and purity of iNK T cells before and after MACS separation, and after a single antigenic expansion (Figure 1B-E). We obtained an average (median, hereafter) of 1.14×105 (range: 948 to 1.20×106) iNK T cells from the entire leukopak after MACS separation, and subsequently acquired a 2.18 × 107 (range: 7.2 × 105 to 5.75 × 107) iNK T cells after an antigenic stimulation. The total numbers of PBMC used to isolate iNK T cells were substantially different among donors ranging from 2.2×108 to 1×109. Therefore, we normalized the absolute numbers of freshly isolated or expanded iNK T cells per 5×108 PBMC in order to accurately estimate the final iNK T cell number (Figure 1D). When normalized, 1.01×105 (range: 1823 to 1.20×106) iNK T cells were obtained from 5×108 PBMC after MACS separation, and 2.80 × 107 (range: 1.8× 106 to 1.7 × 108) iNK T cells were acquired after an antigenic stimulation. The purity of iNK T cells increased from 0.19% (range: 0.02% to 2.59%) pre-MACS separation to 31.96% (range: 0.06% to 96.27%) post MACS separation, and to 98.82% (range: 7.01% to 99.92%) after a single round of antigen specific expansion. There were three donors who failed to expand iNK T cells greater than 88% purity – with 7.01%, 20.17%, and 46.53% respectively. The poor purity of iNK T cells resulted from the inefficient expansion can be further increased to a greater than 95% via an additional MACS purification step (data not shown). Finally, a single antigenic stimulation of enriched iNK T cells resulted in 156.2 folds of expansion (range: 22 to 13186). Interestingly, we observed a trend towards better expansion when a lower number of iNK T cells were present in the donors, but this was not statistically significant (r=−0.4342, p=0.1595).
In summary, we demonstrated it is feasible to reliably expand highly pure iNK T cells to a clinically meaningful number using a single antigen stimulation of iNK T cells that were enriched from PBMC.
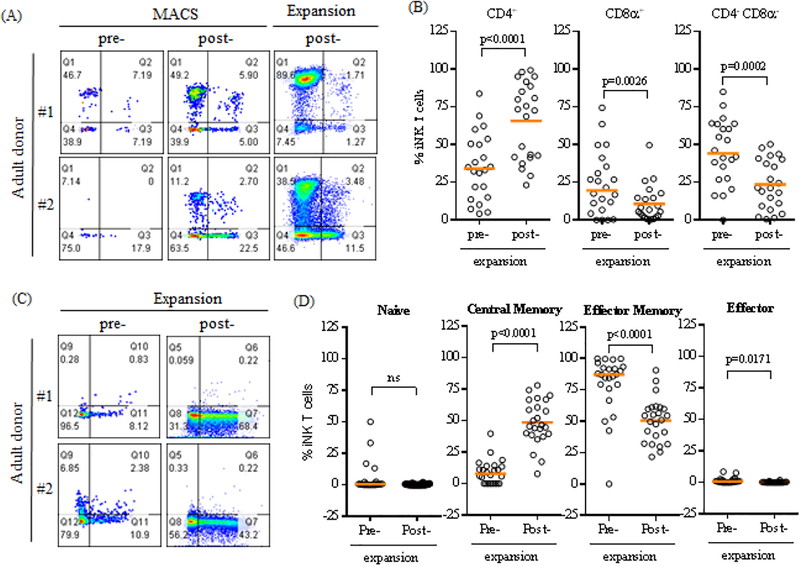
Antigen specific stimulation leads to preferential expansion of CD4+ iNK T cells.
Although both CD4+ and CD4− iNK T cells have overlapping effector-regulator functions, the subtle functional difference may be present between CD4+ and CD4− iNK T cells [24–27]. For example, CD4+ iNK T cells can express a higher level of CD25 and FoxP3 in addition to producing more Th-2 type cytokines than CD4− iNK T cells. Contrary, CD4− iNK T cells can produce more Th-1type cytokines than CD4+ iNK T cells, and have stronger cytotoxic activity. In ASCT, it appears both CD4+ and CD4− iNK T cells may contribute to the immune-regulation. For example, higher donor graft content of CD4− iNK T cells correlates to the lower incidence of GVHD[5, 15]. In addition adoptive transfer of CD4+ iNK T cells prevents murine GVHD [10]. Therefore, we carefully examined the phenotype of expanded iNK T cells from healthy donors. There was a large variation in the percentage of CD4+ iNK T cells prior to the expansion, which was expected from a large donor-to-donor variation of CD4+ iNK T cells present in adult donors[23, 27](Figure 2A-B). To our surprise, antigenic stimulation using αGalCer loaded DC in the presence of IL-2 preferentially expanded CD4+ iNK T cells from 32.8% (range: 10.9% to 64.9%) to 68.8% (range: 37.3% to 87.3%)(Figure 2B), with reciprocal decreases of CD4−CD8α+ or CD4−CD8α− iNK T cells.
Figure 2.
A single antigen stimulation preferentially expand CD4+ and CD45RA−CD62L+ iNK T cells. (A) The representative flow cytometric analysis of CD4 and CD8α expression on iNK T cells pre- and post- enrichment, and post- expansion. (B) The percent CD4+, CD8α+, CD4−CD8α− iNK T cells pre- and post- expansion. (C) The representative flow cytometric analysis of CD45RA and CD62L on iNK T cells pre- and post- expansion. (D) The percent Naïve (CD45RA+CD62L+), central memory (CM, CD45RA−CD62L+), effector memory (EM, CD45RA−CD62L−), and effector (CD45RA+CD62L−) iNK T cells pre and post expansion. Antigen specific stimulation increased % CD4+ iNK T cells from average 34.1 % (range 4.2 % to 83.8 %) to 73.0 % (range:23.2 % to 99.3 %), with reciprocal decreases in % CD8α+ or CD4-CD8α− iNK T cells. In adult donors, the majority of iNK T cells were effector memory phenotype in average 87.2 % (range 0 % to 100 %). After antigenic stimulation, central memory iNK T cells significantly increased from 7.8 % (range: 0 % to 39.8 %) to 46.0 % (range: 7.8 % to 78.1%), with reciprocal decreased in effector memory phenotype to 52.8 % (range: 21.7 % to 90.7 %). A single symbol represents a value from a single donor. The median was used as average value. A paired student t-test was used to compare the differences between selected groups. P-value less than 0.05 was considered as “significant”
Recently, CD62L+ human iNK T cells have been shown to persist better in vivo, and to display better anti-tumor activity than CD62L− iNK T cells when genetically modified to target tumor antigens [28]. Thus, we carefully assessed the change of memory phenotype of iNK T cells at pre- and post ex vivo expansion (Figure 2C-D). The major memory phenotype of iNK T cells present in the peripheral bloods of adult donors were effector memory (EM, CD45RACD62L−) phenotypes with 87.2 % (range 0 % to 100 %), and central memory (CM, CD45RACD62L+) iNK T cells constituted the minor population with 7.8 % (range: 0 % to 39.8 %). Interestingly, an antigenic stimulation promoted the expansion of CM iNK T cells to 46.0 % (range: 7.8 % to 78.1%) with reciprocal decrease of EM iNK T cells to 52.8 % (range: 21.7 % to 90.7 %). Thus, expanded iNK T cells may persist better than freshly isolated iNK T cells.
In summary, we demonstrated that a single antigenic stimulation promotes the preferential expansion of CD4+ iNK T cells and supports the expansion of potentially better persisting CD62L+ iNK T cells
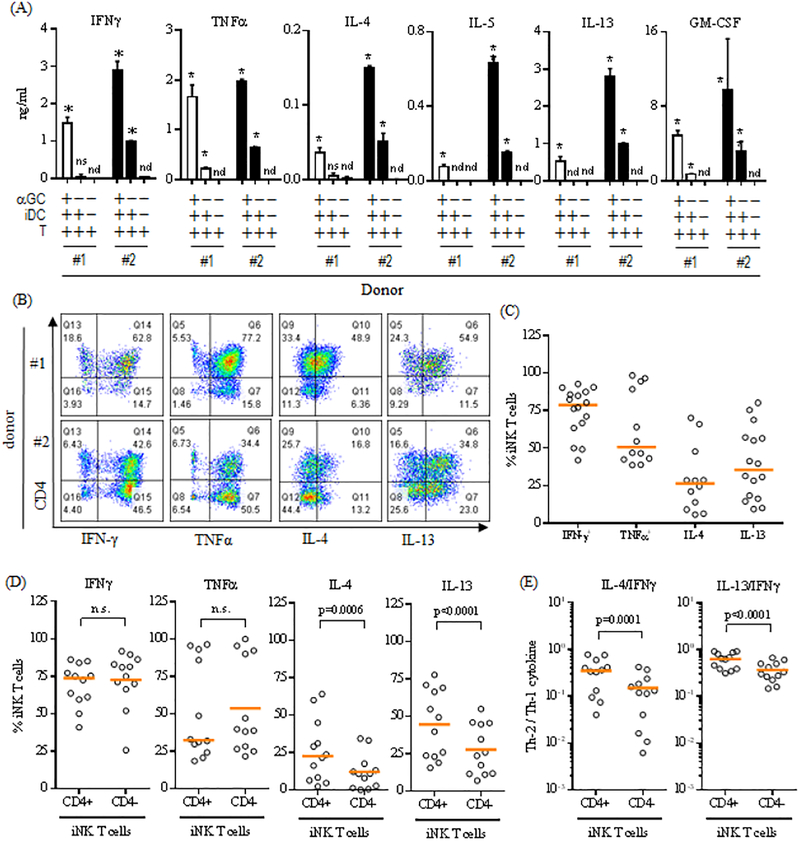
Expanded human iNK T cells maintain the ability to produce both Th-1 and Th-2 type cytokines.
One mechanism by which iNK T cells exert their regulatory functions is through the production of Th-1 or Th-2 type cytokines [24, 25, 27]. Here, we assessed whether expanded iNK T cells maintained the ability to produce both Th-1 and Th-2 type cytokines upon antigen specific stimulation (Figure 3A). The iNK T cells were activated by αGalCer pulsed irradiated DC and the cytokine production profile was assessed from the culture supernatant. As shown in Figure 3A, iNK T cells from two representative donors were able to produce both Th-1 (IFNγ and TNFα) and Th-2 type cytokines (IL-4, IL-5, IL-13, and GM-CSF) although in different quantities. Interestingly, iNK T cells from donor #2 produced lower levels of various cytokines in the absence of an agonist glycolipid during co-culture with DC. This can be explained by known autoreactivity of iNK T cells attributed from the recognition of self or endogenous glycolipid antigens presented by CD1d in antigen presenting cells (APC) [22, 29, 30].
Figure 3.
Expanded human iNK T cells produce both Th-1 and Th-2 type cytokines. (A) The ex vivo expanded iNK T cells were stimulated by DC in the presence or absence of αGalCer for 48 hours, and cytokines present in the culture supernatants were assessed using standard capture ELISA. Cytokine production profiles of iNK T cells from two representative donors among 5 donors tested were shown in (A), and demonstrated that expanded NK T cells produced both Th-2 (IL-4, IL-5, IL-13, and GM-CSF) and Th-1 type cytokines (IFNγ and TNFα). An unpaired student-t test was used to compare the differences of cytokine production with control group, and “*” denotes P-value less than 0.05. n.d.: not detected, n.s.: not significant. (B-D) The expanded iNK T cells were stimulated with PMA (30 ng/ml) and Ionomycin (1 μg/ml) in the presence of monensin (1 μM) for 3 hours and subjected to intracellular cytokine analysis. The representative flow cytometric analyses of intracellular cytokines were shown in (B), the percentages of cytokine+ iNK T cells from the total iNK T cells or CD4+/− iNK T cell subsets were plotted in (C) and (D) respectively, and the ratios of the percent Th-2 cytokine+ CD4+/− iNK T cells to Th-1 cytokine+ CD4+/− iNK T cells were shown in (E). While both CD4+ and CD4− iNK T cells produced a similar level of Th-1 type cytokines (IFNγ and TNFα), significantly larger portion of CD4+ iNK T cells produced Th-2 type cytokines (IL-4 and IL-13) than CD4− iNK T cells (D), and CD4+ iNK T cells produced significantly more Th-2 type cytokines than CD4− iNK T cells as evidenced by significantly higher ratio of Th-2 type cytokine+ iNK T cells to Th-1 type cytokine+ iNK T cells (E). A single symbol represents a value from a single donor. The median was used as average value. A paired student t-test was used to compare the differences between selected groups. P-value less than 0.05 was considered as “significant”
In order to confirm our findings in a larger number of donors (N=16), we chose to assess various intracellular cytokine production from expanded iNK T cells stimulated by phorbol 12-myristate 13-acetate (PMA) and Ionomycin in the presence of monensin (Figure 3B-E), and investigate subtle differences of the cytokine production profile between CD4+ and CD4− iNK T cells as antigenic stimulation tends to preferentially expand CD4+ iNK T cells (Figure 3B-E). As expected, iNK T cells produced both Th-1 and Th-2 type cytokines, however, there was a significant donor to donor variation in terms of ability to produce cytokines (Figure B-C). Agreater portion of iNK T cells produced Th-1 type cytokines – IFNγ (78.6% with range: 41.9% to 92.7%) and TNFα (50.6%, range: 48.6% to 98.4%), while a smaller fraction of iNK T cells were able to produce Th-2 type cytokines – IL-4 (26.4%, range: 5.6% to 70.2%) and IL-13 (35.4%, range:9.2% to 80.2%). As anticipated from previous observation[24–27], there was a subtle difference in terms of the cytokine production profile between CD4+ and CD4− iNK T cells (Figure 3D-E). While CD4+ and CD4− iNK T cells produced Th-1 type cytokines (IFNγ and TNFα) at a similar level, significantly larger percentages of CD4+ iNK T cells produced Th-2 type cytokines (IL-4 and IL-13) compared to CD4− iNK T cells (Figure 3D). This was translated into a significantly higher ratio of Th-2 type cytokine+ iNK T cells to Th-1 type cytokine+ iNK T cells in CD4+ iNK T cells compared to CD4− iNK T cells (Figure 3E).
In summary, our results suggest that expanded iNK T cells maintain the intrinsic ability to produce both Th-1 and Th-2 type cytokines with subtle polarization towards Th-2 type cytokine production profile in CD4+ iNK T cells.
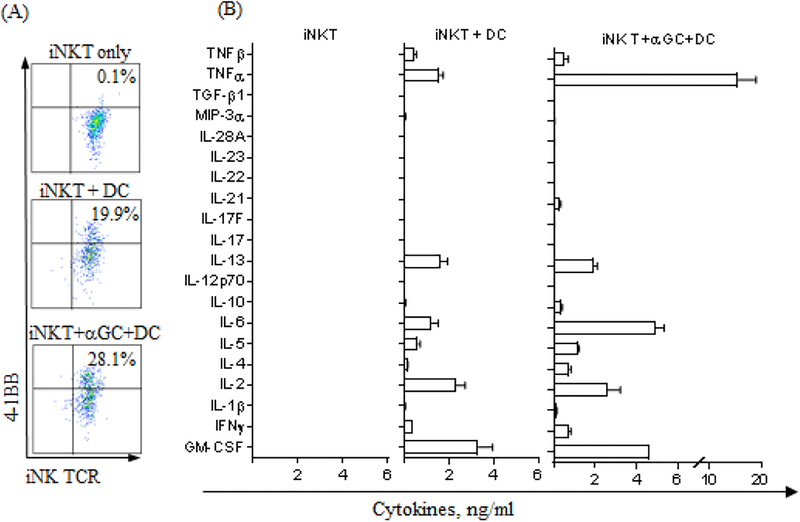
Expanded iNK T cells are highly autoreactive.
We observed that expanded iNKT cells produced a considerate amount of cytokines when co-cultured with DC in the absence of αGalCer, and this is likely due to the known autoreactivity through the recognition of endogenous antigens in the context of CD1d [29, 31](Fig 3A). Thus, we investigated the comprehensive cytokine production profile of autoreactive iNK T cells in comparison to iNK T cells stimulated by cognate antigens, which may be more relevant to the novel mechanism of the immune-regulation in allogeneic stem cell transplantation.
The iNK T cells were stimulated by DC with or without αGalCer for 16–48 hours, then evaluated for the expression of 4–1BB, activation marker, and comprehensive 20 Th-1/Th-2/Th-17 panel cytokines from the culture supernatants. The subset of iNK T cells upregulated 4–1BB upon co-culture with DC alone, and percent 4–1BB+ iNK T cells further increased when αGalCer was added to co-culture (Fig 4A). These results supported that expanded iNK T cells can be activated by dendritic cells in an autoreactive manner. Interestingly, iNK T cells stimulated by DC produced both Th-1 and Th-2 type cytokines albeit to a lesser degree compared to when activated by cognate antigen (Figure 2B). Strikingly, expanded iNK T cells fully stimulated by cognate antigen pulsed DC produced a significantly larger amount of Th-1 cytokine, TNFα. Hense the quality of antigenic stimulation may impact the functional polarization of activated iNK T cells. Our findings suggest that autoreactive iNK T cells may preferentially produce Th-2 type cytokines, implicating a novel mechanism of the immune-regulation to control GVHD in ASCT as well as several autoimmune diseases.
Figure 4.
Expanded iNK T cells are highly autoreactive. Ex vivo expanded iNK T cells were stimulated by dendritic cells in the presence of absence of αGalCer for 16–48 hours. Activated iNK T cells were assessed for activation marker, 4–1BB after 16 hours of stimulation (A) and culture supernatants after 48 hours’ stimulation were used for comprehensive cytokine analysis (B). Significant portion of iNK T cells co-cultured with dendritic cells in the absence of agonist antigen, αGalCer, upregulated expression of activation marker, 4–1BB, and the percent 4–1BB+ iNK T cells were further increased with the addition of agonist antigen, αGalCer to co-culture Further, expanded iNK T cells produced both Th-1 and Th-2 type cytokines when co-cultured with dendritic cells only, and the production of various cytokines, especially Th-1 type cytokines such as TNFa, was significantly augmented when co-cultured with dendritic cells in the presence of αGalCer.
Highly pure iNK T cells ameliorate xenograft GVHD.
CD4+CD25+FoxP3+ regulatory T cells can inhibit the proliferation of alloreactive T cells in a mixed lymphocyte reaction (MLR) [32]. Here, we evaluated whether expanded iNK T cells can suppress the proliferation of conventional T cells (Figure 5A). Instead of MLR, we chose to utilize the activated T cells via soluble anti-CD3 and CD28 antibodies as responders because the proliferation of responder T cells was more reproducible and predictable in our hands. As shown in Fig 5A and B, the stimulation with anti-CD3/CD28 antibodies efficiently activated T cells resulting in explosive proliferation. The addition of expanded iNK T cells, but not the control allogeneic T cells, significantly inhibited the proliferation of conventional T cells at a ratio of 10 responder T cells to 1 iNK T cell. However, iNK T cells did not completely abolish the proliferation of conventional T cells, and this partial inhibition is likely due to strong T cell receptor (TCR) mediated stimulation of responder T cells through anti-CD3/CD28 antibodies or relatively high but rather physiologic ratio of responder T cells to iNK T cells. We did not observe the significant correlation between the percentages of CD4+ iNK T cells to the immunosuppressive function, likely due to the presence of overlapping suppressive function in CD4− iNK T cells as previously described by Chaidos et al[5].
Figure 5.
Expanded iNK T cells maintain in vitro immunosuppressive function. CFSE-labeled conventional T cells (TCFSE) were stimulated with soluble anti-CD3 and anti-CD28 antibodies, then iNK T cells were added at 10 TCFSE to 1 iNK T cell. The flow cytometric analysis of the diluted CFSE of TCFSE was performed as a measurement of proliferation after 5 days of co-culture. The representative flow cytometric analysis of CFSE of TCSFE in immunosuppression assay was shown in (A) and the percentage of proliferation in (B). The expanded iNK T cells, but not control T cells, were able to significantly inhibit the αCD3/CD28 antibody-induced proliferation of responder T cells. The results from one of 8 donors were shown. An unpaired student t-test was used to assess the differences in proliferation compared to the control group.
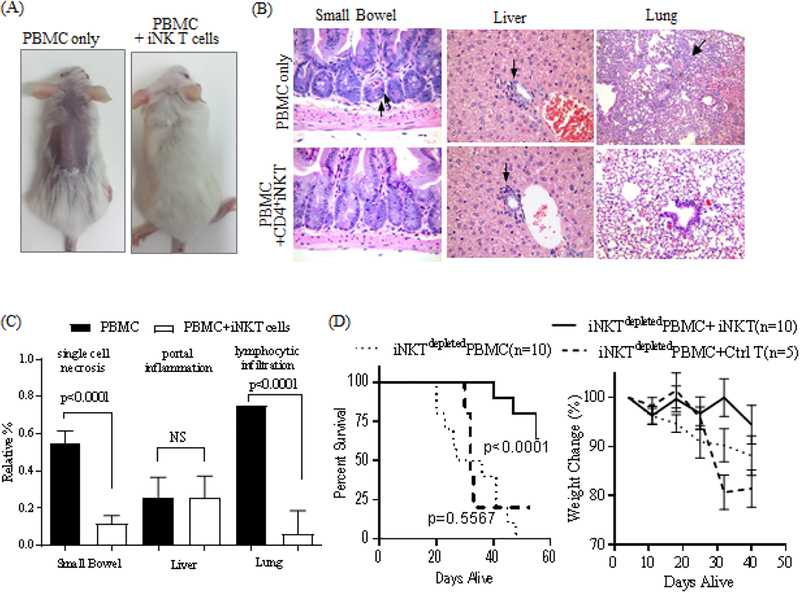
Next, we investigated whether the expanded iNK T cells displayed an immunosuppressive function in vivo using a well-established xenograft GVHD murine model [32, 33]. Highly pure, and predominantly CD4+ iNK T cells from two donors were chosen for two independent in vivo experiments. We decided to deplete iNK T cells from PBMC to elicit xenograft GVHD more consistently, and to avoid confounding effects from iNK T cells present in PBMC. As previously reported, NSG mice infused with human iNKTdepleted PBMC quickly developed clinical signs of GVHD (hair loss, decreased activity, kyphosis, and weight loss) as well as pathologic signs of GVHD represented by the increased single cell necrosis in the GI tract and lymphocyte infiltration in the lung and liver (Figure 6A-C). In contrast, NSG mice co-infused with human iNKTdepleted PBMC and iNK T cells (ratio of 1 iNK T cells to 20 iNKTdepleted PBMC) had delayed clinical GVHD and significantly lesser degrees of single cell necrosis in the GI tract and lymphocyte infiltration in the lung, resulting in an improved survival at day 56 (Hazard Ratio 0.1266 (95% Confidence Interval: 0.02234 – 0.2413), p<0.0001). (Figure 6A-D). Although there was clear pathologic evidence of tissue inflammation and damage on day 7 of xenograft shown in Figure 6B and 6C, we did not detect proinflammatory cytokines such as IL-6, IFNγ or IFNα in sera of xenografted mice or increased frequencies of CD4+CD25+CD127low regulatory T cells in NSG mice xenografted with PBMC and iNK T cells (data not shown), and this is likely due to early time point of analysis. Thus, our results suggest that ex vivo expanded iNK T cells maintained in vitro and in vivo immunosuppressive activity suited for novel cell therapy to prevent GVHD.
Figure 6.
Expanded iNK T cells maintain in vivo immunosuppressive function. Sub-lethally irradiated NSG mice received 1×107 human iNKTdepeted PBMC with or without 5×105 iNK T cells or allogeneic control T cells, and were assessed for developing clinical and pathologic GVHD. NSG mice infused with human iNKTdepleted PBMC quickly developed evidences of clinical GVHD (A)(hunched back, hair loss, and weight loss) as well as pathologic GVHD (BC)(increased cellular necrosis in GI track, portal inflammation, and lymphocytic infiltration in the lung). In contrast, mice co-infused with iNK T cells did not show signs of clinical GVHD and ameliorated pathologic GVHD, resulting in significantly improved overall survival (D). The difference in animal survival was estimated by log-rank test. Results were combined from two independent experiments. An unpaired student t-test was used to compare differences in pathologic findings between groups. P-value less than 0.05 was considered as “significant”
Discussion
Previous exploration of iNK T cells in immunotherapy have mostly focused on their potential anti-tumor effects [34–37]. Vaccination strategies using αGalCer-loaded autologous dendritic cells have been employed in certain malignancies such as multiple myeloma and squamous cell carcinoma of the head and neck with promising results, and most recently chimeric antigen receptor (CAR)-transduced iNK T cells have been developed for the treatment of medulloblastoma in a preclinical study [34–37]. Despite the clear potential of iNK T cells to modulate the immune microenvironment in allogeneic stem cell transplantation (ASCT) as well as various autoimmune diseases, adoptive cell therapy using iNK T cells to promote immune-regulation has not been successfully performed in a clinical setting.. This is because iNK T cells are present in very low frequency and enormous variability in their numbers in normal human blood [23]. Thus, it is difficult to acquire sufficient numbers of highly immunosuppressive iNK T cells. Therefore, an effective strategy to reliably obtain iNK T cells in clinically meaningful numbers is needed for the development of the iNK T cell based immunotherapy to promote immune-regulation.
Unlike conventional regulatory T cells which constitute 5% of circulating T cells, iNK T cells are under tight homeostasis and comprise less than 0.1% of T cells. Nevertheless, they are powerful immune-regulators. For example, the presence of greater than 4×104 to 1×105 per kg of iNK T cells in the donor grafts is associated with a lower incidence of acute GVHD in the recipients after ASCT [5, 16]. This observation enables us to estimate 4×106 to 1×107 iNK T cells as a clinically meaningful number enough to supplement donor graft for the prevention of GVHD. Therefore, we aimed to develop a strategy to expand human iNK T cells to this “clinically meaningful number” in minimum production time. With a combination of magnetic bead based one-step enrichment and highly effective antigen specific stimulation, we obtained 2.8 × 107 (range: 1.8× 106 to 1.7 × 108) from 5×108 PBMC in extremely high purity (98.8%) within 2 weeks of preparation for 24 consecutive adult donors. Only 1 out of the 24 consecutive adult donors failed to reach to more than 4×106 iNK T cells. Thus, it is feasible to acquire highly pure iNK T cells to a clinically meaningful number in 2 weeks via a single antigenic stimulation.
The distinct features of our approach to expand iNK T cells include the upfront use of MACS separation using commercially available anti-NKT microbeads and subsequent antigenic stimulation incorporating monocyte-derived allogeneic dendritic cells as highly efficient antigen presenting cells. The use of MACS separation to enrich specific immune subsets prior to the ex vivo expansion has been used to develop adoptive cell therapy with human regulatory T cells [32, 38]. Here, we provide another example of the successful application of MACS separation. This initial enrichment using MACS separation was found to be a crucial step to overcome the extremely scarcity of iNK T cells in a large donor-to-donor variation, and resulted in reliable expansion in such high purity.
Since iNK T cells recognize glycolipid antigens presented by the non-classical antigen presenting protein, CD1d, which is evolutionally conserved without polymorphism, we opted to use monocyte-derived dendritic cells from allogeneic donors. This approach enables us to cryopreserve highly effective monocyte-derived dendritic cells as universal antigen presenting cells (APC) to expand iNK T cells from multiple donors, and confers major practical advantage by eliminating the need to prepare autologous APC for each donor. In addition, the functional modulation of iNK T cells towards Th-1, Th-2, or even Th-17 may be feasible during ex vivo expansion by incorporating various Th-1, Th-2, or Th-17 polarizing cytokines[39–41] and Th-1 or Th-2 polarizing αGalCer analogues[42, 43]. Therefore, one can accentuate certain properties of expanded iNK T cells such as regulatory function for immune-suppression or effector functions for anti-tumor activity which are appropriate to treat corresponding diseases such as autoimmune diseases and graft versus host disease in transplantation or malignancies, respectively.
Although both CD4+ and CD4− iNK T cells can lyse CD1d+ tumor cells and produce Th-1 and Th-2 type cytokines, there is a subtle difference in the dominant function of CD4 positive and negative subsets of iNK T cells[23–27]. For example, CD4+ iNK T cells can express CD25 and FoxP3 as well as produce more Th-2 type cytokines than CD4− iNK T cells. In contrast, CD4− iNK T cells can exert better cytolytic functions and express higher levels of perforin, granzyme, chemokine receptors, and activating natural killer receptors than CD4+ iNK T cells. Therefore, one can speculate that CD4+ iNK T cells may be a better regulator while CD4− iNK T cells be better effectors. The cytokine production profile of expanded iNK T cells confirmed this subtle Th-1 vs Th-2 polarization as CD4+ iNK T cells were indeed polarized towards Th-2-type cytokine responses compared to CD4− iNK T cells. In addition, our protocol preferentially expanded CD4+ iNK T cells to greater than 70% for the majority of adult donors and these highly enriched CD4+ iNK T cells displayed in vivo immunosupressive functions to ameliorate xenogenic GVHD (Hazard Ratio 0.1266, (95% Confidence Interval: 0.02234 – 0.2413), p<0.0001). Thus, our expansion strategy may be a suitable to prepare iNK T cells for adoptive cell therapy to promote immune-suppression.
In ASCT, the relative contribution of CD4+ or CD4− iNK T cells to the immune-regulation is the subject of an on-going discussion as preclinical and clinical studies currently support both CD4+ and CD4− iNK T cells for their regulatory role in preventing GVHD[5, 9–16]. This is likely because both CD4+ and CD4− iNK T cells are able to inhibit conventional T cell proliferation (Figure 5A)[5] and to produce Th-2 type regulatory cytokines (Figure 2). However, enriched CD4+ iNK T cells may be better suited for autologous adoptive cell therapy to supplement donor grafts to promote immune-regulation in ASCT, as there is subtle Th-2 polarization of ex vivo expanded CD4+ iNK T cells. Furthermore, one can establish a tissue bank where highly enriched ex vivo expanded CD4+ iNK T cells are prepared from the selected donors. As the third party CD4+ iNK T cells are known to prevent acute and chronic GVHD through the expansion of conventional Treg in murine GVHD models[11, 12], these highly enriched CD4+ iNK T cells can be used as the third party adoptive cellular therapy to enhance immune-regulation not only in ASCT but also in solid organ transplantation as well as other autoimmune diseases.
Acknowledgement
This work is supported by 5-P01-CA 148600–04 (J.M.), 2-P50-CA100632–12 (J.M, E.J.S), RO1 AI45889 (S.A.P), T32-CA009666 (J.I), MD Anderson Advanced Scholar (J.I.), New Investigator Award from American Society of Blood and Marrow Transplantation (J.I.). Amy Strelzer Manasavit Research Scholar from National Marrow Donor Program (J.I.), and MD Anderson Institutional Start-Up (J.I). The South Campus Flow Cytometry & Cell Sorting Core is supported by NCI P30CA016672. Lastly, we thank Ms Annalea Elwell for proof-reading manuscript.
Abbreviation
- ASCT
allogeneic hematopoietic stem cell transplantation
- iNKT
invariant Natural Killer
- GVHD
graft versus host disease
- GVL
graft verus leukemic effects
- MACS
Magnetic Activated Cell Sorting
- DC
dendritic cells
- αGalCer
alpha-galactosyl ceramide
- PBMC
peripheral blood mononuclear cells
- CM
central memory
- EM
effector memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Holowiecki J, Indications for hematopoietic stem cell transplantation, Polskie Archiwum Medycyny Wewnetrznej 118(11) (2008) 658–63. [PubMed] [Google Scholar]
- [2].Zander AR, Bacher U, Finke J, Allogeneic stem cell transplantation in acute myeloid leukemia: establishment of indications on the basis of individual risk stratification, Deutsches Arzteblatt international 105(39) (2008) 663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferrara JL, Levine JE, Reddy P, Holler E, Graft-versus-host disease, Lancet 373(9674) (2009) 1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benlagha K, Bendelac A, CD1d-restricted mouse V alpha 14 and human V alpha 24 T cells: lymphocytes of innate immunity, Seminars in immunology 12(6) (2000) 537–42. [DOI] [PubMed] [Google Scholar]
- [5].Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, McDonald D, Marin D, Milojkovic D, Pavlu J, Davis J, Rahemtulla A, Rezvani K, Goldman J, Roberts I, Apperley J, Karadimitris A, Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation, Blood 119(21) (2012) 5030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hongo D, Tang X, Dutt S, Nador RG, Strober S, Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants, Blood 119(6) (2012) 1581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karadimitris A, Chaidos A, The role of invariant NKT cells in allogeneic hematopoietic stem cell transplantation, Critical reviews in immunology 32(2) (2012) 157–71. [DOI] [PubMed] [Google Scholar]
- [8].Pillai AB, George TI, Dutt S, Strober S, Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease, Blood 113(18) (2009) 4458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuns RD, Morris ES, Macdonald KP, Markey KA, Morris HM, Raffelt NC, Banovic T, Don AL, Rowe V, Burman AC, Clouston AD, Farah C, Besra GS, Illarionov PA, Smyth MJ, Porcelli SA, Hill GR, Invariant natural killer T cell-natural killer cell interactions dictate transplantation outcome after alpha-galactosylceramide administration, Blood 113(23) (2009) 5999–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schneidawind D, Pierini A, Alvarez M, Pan Y, Baker J, Buechele C, Luong RH, Meyer EH, Negrin RS, CD4+ invariant natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells, Blood 124(22) (2014) 3320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schneidawind D, Baker J, Pierini A, Buechele C, Luong RH, Meyer EH, Negrin RS, Third-party CD4+ invariant natural killer T cells protect from murine GVHD lethality, Blood 125(22) (2015) 3491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Du J, Paz K, Thangavelu G, Schneidawind D, Baker J, Flynn R, Duramad O, Feser C, Panoskaltsis-Mortari A, Negrin RS, Blazar BR, Invariant natural killer T cells ameliorate murine chronic GVHD by expanding donor regulatory T cells, Blood (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duramad O, Laysang A, Li J, Ishii Y, Namikawa R, Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models, Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 17(8) (2011) 1154–68. [DOI] [PubMed] [Google Scholar]
- [14].Rubio MT, Moreira-Teixeira L, Bachy E, Bouillie M, Milpied P, Coman T, Suarez F, Marcais A, Sibon D, Buzyn A, Caillat-Zucman S, Cavazzana-Calvo M, Varet B, Dy M, Hermine O, Leite-de-Moraes M, Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival, Blood 120(10) (2012) 2144–54. [DOI] [PubMed] [Google Scholar]
- [15].Rubio MT, Bouillie M, Bouazza N, Coman T, Trebeden-Negre H, Gomez A, Suarez F, Sibon D, Brignier A, Paubelle E, Nguyen-Khoc S, Cavazzana M, Lantz O, Mohty M, Urien S, Hermine O, Pre-transplant donor CD4- invariant NKT cell expansion capacity predicts the occurrence of acute graft-versus-host disease, Leukemia (2016). [DOI] [PubMed] [Google Scholar]
- [16].Malard F, Labopin M, Chevallier P, Guillaume T, Duquesne A, Rialland F, Derenne S, Peterlin P, Leaute AG, Brissot E, Gregoire M, Moreau P, Saas P, Gaugler B, Mohty M, Larger number of invariant natural killer T cells in PBSC allografts correlates with improved GVHD-free and progression-free survival, Blood 127(14) (2016) 1828–35. [DOI] [PubMed] [Google Scholar]
- [17].Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC, Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells, J Immunol 167(6) (2001) 3114–22. [DOI] [PubMed] [Google Scholar]
- [18].Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS, Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages, The Journal of clinical investigation 119(6) (2009) 1524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taniguchi M, Harada M, Dashtsoodol N, Kojo S, Discovery of NKT cells and development of NKT cell-targeted anti-tumor immunotherapy, Proc Jpn Acad Ser B Phys Biol Sci 91(7) (2015) 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang YT, Illarionov PA, Besra GS, Porcelli SA, Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation, Immunity 30(6) (2009) 888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA, Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides, Proceedings of the National Academy of Sciences of the United States of America 102(9) (2005) 3383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Im JS, Tapinos N, Chae GT, Illarionov PA, Besra GS, DeVries GH, Modlin RL, Sieling PA, Rambukkana A, Porcelli SA, Expression of CD1d molecules by human schwann cells and potential interactions with immunoregulatory invariant NK T cells, J Immunol 177(8) (2006) 5226–35. [DOI] [PubMed] [Google Scholar]
- [23].Im JS, Kang TJ, Lee SB, Kim CH, Lee SH, Venkataswamy MM, Serfass ER, Chen B, Illarionov PA, Besra GS, Jacobs WR Jr., Chae GT, Porcelli SA, Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections, Clinical immunology 127(2) (2008) 214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O’Reilly V, Zeng SG, Bricard G, Atzberger A, Hogan AE, Jackson J, Feighery C, Porcelli SA, Doherty DG, Distinct and overlapping effector functions of expanded human CD4+, CD8alpha+ and CD4-CD8alpha-invariant natural killer T cells, PloS one 6(12) (2011) e28648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee PT, Benlagha K, Teyton L, Bendelac A, Distinct functional lineages of human V(alpha)24 natural killer T cells, The Journal of experimental medicine 195(5) (2002) 637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gumperz JE, Miyake S, Yamamura T, Brenner MB, Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining, The Journal of experimental medicine 195(5) (2002) 625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chan AC, Leeansyah E, Cochrane A, d’Udekem d’Acoz Y, Mittag D, Harrison LC, Godfrey DI, Berzins SP, Ex-vivo analysis of human natural killer T cells demonstrates heterogeneity between tissues and within established CD4(+) and CD4(−) subsets, Clinical and experimental immunology 172(1) (2013) 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, Guo L, Xu X, Torikai H, Mo Q, Dotti G, Cooper LJ, Metelitsa LS, CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo, The Journal of clinical investigation 126(6) (2016) 2341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Exley M, Garcia J, Balk SP, Porcelli S, Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8-T cells, The Journal of experimental medicine 186(1) (1997) 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB, Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals, Nature immunology 12(12) (2011) 1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Exley M, Porcelli S, Furman M, Garcia J, Balk S, CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains, The Journal of experimental medicine 188(5) (1998) 867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parmar S, Liu X, Tung SS, Robinson SN, Rodriguez G, Cooper LJ, Yang H, Shah N, Yang H, Konopleva M, Molldrem JJ, Garcia-Manero G, Najjar A, Yvon E, McNiece I, Rezvani K, Savoldo B, Bollard CM, Shpall EJ, Third-party umbilical cord blood-derived regulatory T cells prevent xenogenic graft-versus-host disease, Cytotherapy 16(1) (2014) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parmar S, Liu X, Najjar A, Shah N, Yang H, Yvon E, Rezvani K, McNiece I, Zweidler-McKay P, Miller L, Wolpe S, Blazar BR, Shpall EJ, Ex vivo fucosylation of third-party human regulatory T cells enhances anti-graft-versus-host disease potency in vivo, Blood 125(9) (2015) 1502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, Miesowicz F, Dhodapkar KM, Dhodapkar MV, Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma, Blood 121(3) (2013) 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurosaki M, Horiguchi S, Yamasaki K, Uchida Y, Motohashi S, Nakayama T, Sugimoto A, Okamoto Y, Migration and immunological reaction after the administration of alphaGalCer-pulsed antigen-presenting cells into the submucosa of patients with head and neck cancer, Cancer immunology, immunotherapy: CII 60(2) (2011) 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, Shimizu N, Ueno N, Yamamoto S, Taniguchi M, Motohashi S, Nakayama T, Okamoto Y, Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy, Clinical immunology 138(3) (2011) 255–65. [DOI] [PubMed] [Google Scholar]
- [37].Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, Liu H, Dotti G, Metelitsa LS, Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy, Blood (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chakraborty R, Mahendravada A, Perna SK, Rooney CM, Heslop HE, Vera JF, Savoldo B, Dotti G, Robust and cost effective expansion of human regulatory T cells highly functional in a xenograft model of graft-versus-host disease, Haematologica 98(4) (2013) 533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, Metelitsa LS, Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans, Blood 104(13) (2004) 4150–6. [DOI] [PubMed] [Google Scholar]
- [40].Monteiro M, Almeida CF, Agua-Doce A, Graca L, Induced IL-17-producing invariant NKT cells require activation in presence of TGF-beta and IL-1beta, Journal of immunology 190(2) (2013) 805–11. [DOI] [PubMed] [Google Scholar]
- [41].Moreira-Teixeira L, Resende M, Coffre M, Devergne O, Herbeuval JP, Hermine O, Schneider E, Rogge L, Ruemmele FM, Dy M, Cordeiro-da-Silva A, Leite-de-Moraes MC, Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells, Journal of immunology 186(10) (2011) 5758–65. [DOI] [PubMed] [Google Scholar]
- [42].Bricard G, Venkataswamy MM, Yu KO, Im JS, Ndonye RM, Howell AR, Veerapen N, Illarionov PA, Besra GS, Li Q, Chang YT, Porcelli SA, Alpha-galactosylceramide analogs with weak agonist activity for human iNKT cells define new candidate anti-inflammatory agents, PloS one 5(12) (2010) e14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lu X, Song L, Metelitsa LS, Bittman R, Synthesis and evaluation of an alpha-C-galactosylceramide analogue that induces Th1-biased responses in human natural killer T cells, Chembiochem 7(11) (2006) 1750–6. [DOI] [PubMed] [Google Scholar]