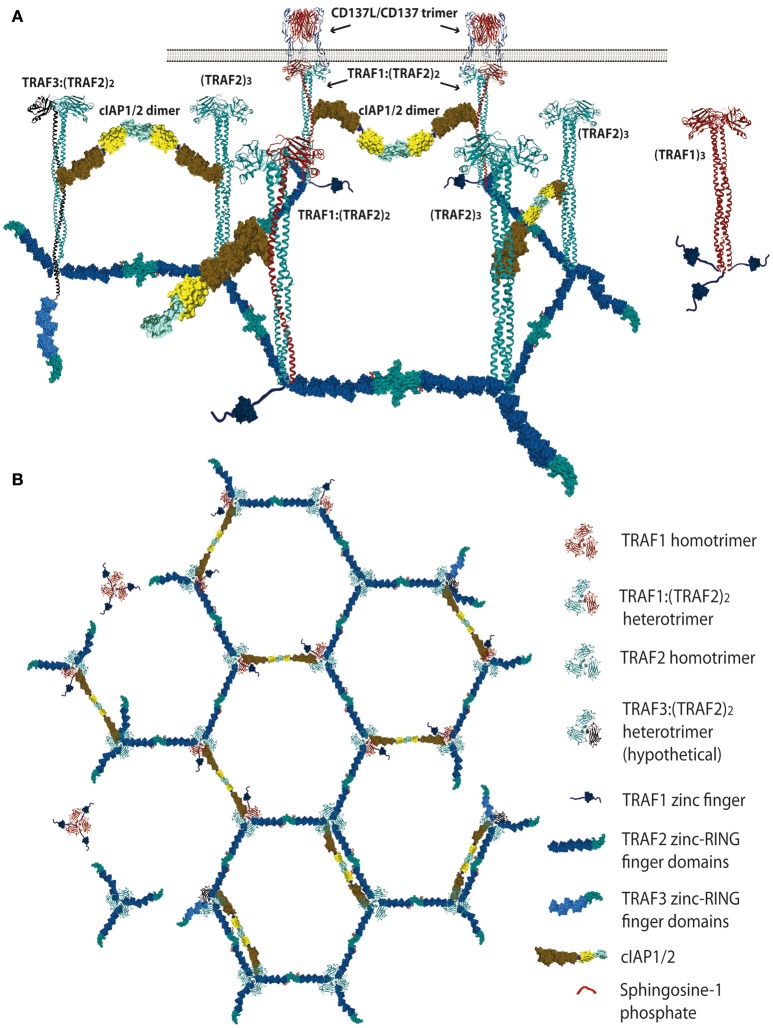
Figure 1.
Schematic representation of the proposed TRAF trimer configurations and interactions in the CD137L/CD137 hexagonal lattice. (A) Lateral view representing the various TRAF-trimer configurations that could be recruited to the activated CD137 trimers. The figure also shows the TRAF2-RING finger dimers that would likely be formed between the RING finger domains of two TRAF2 molecules from adjacent trimers, which is a requirement for E3 ubiquitin ligase activity. Similar interactions between the RING domains of cIAP1/2 from contiguous trimers are also expected. (B) It is show in top view how the CD137-recruited TRAF trimers would arrange forming a large hexagonal network that would be stabilized by the establishment of RING finger domains dimers between the TRAF2 molecules from adjacent trimers or between the RING finger domains of contiguous cIAP1/2 molecules. Further explanation in the text. Protein structure coordinates were obtained from the PDB database and molecular graphics were performed with UCSF Chimera (15).