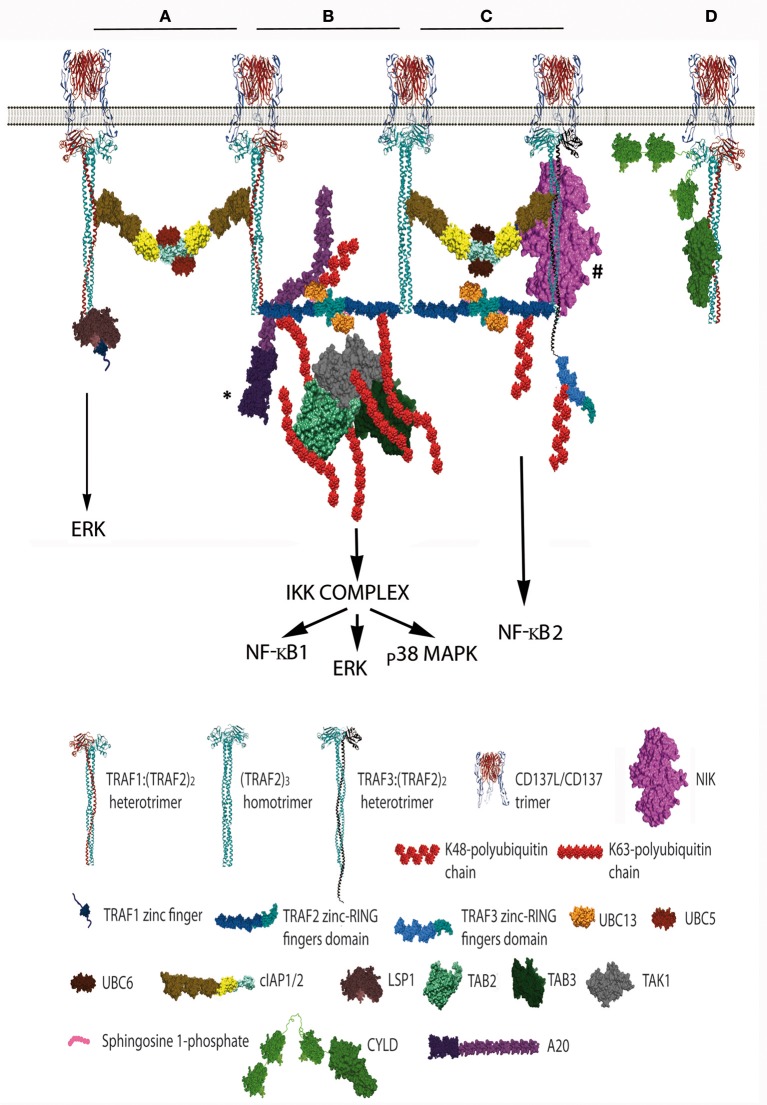
Figure 2.
Schematic representation of the distinct CD137 signalosomes that would be formed upon CD137 activation. This figure illustrates the distinct signalosomes that could be formed in response to CD137 activation depending on the TRAF trimer configurations that get associated to the activated CD137. (A) cIAP1/2 bridging between 2 TRAF1(TRAF2)2 trimers. What other molecules, besides E2 proteins, would be specifically recruited to this configuration is yet unknown. The binding of Lymphocyte specific protein-1 to the N-terminal region of TRAF1 is shown. (B) The formation of a dimer between the RING finger domains of 2 TRAF2 molecules from adjacent trimers will trigger K63 ubiquitination of TRAF2 and the subsequent recruitment and activation of the TAK1/TAB1/TAB2/TAB3 complex (TAB1 is not shown). K63-TAK1-mediated IKKβ phosphorylation will activate the IKK complex activation initiating a signaling cascade that will result in NF-κB1 and ERK activation. A20 might inhibit this signaling cascade by K48-ubiquitinating Ubc13 thus inhibiting TRAF2 E3 ubiquitin ligase activity. * A20 can form dimers, but a sole A20 molecule is represented for clarity. (C) Hypothetical organization of a signalosome that includes a TRAF3:(TRAF2)2 trimer. The cIAP1/2 molecules associated either to a TRAF2 homotrimer and the hypothetical TRAF3:(TRAF2)2 trimer will form a dimer by the interaction of their RING fingers domains causing the activation of the E3 ubiquitin ligase activity. Thus, the cIAP1/2 dimer will K48-ubiquitinate TRAF3 and TRAF2 molecules targeting them for proteasome degradation and effectively releasing NIK from its interaction with TRAF3, resulting in the activation of NF-κB2 as has been observed following CD137 stimulation. # The TRAF region binding to NIK is still controversial, since reports indicating that is mediated by either the TRAF domain (56, 57) or the RING-zinc finger region (58, 59) are available. (D) CYLD interacts with the same crevice in the TRAF domain that binds to CD137 cytosolic tail. CYLD might works as a gate keeper preventing ligand-independent TRAF activation but it might also participate in the termination of CD137 signaling by outcompeting CD137 from binding to TRAF2 as shown in the figure. Further explanation in the text. Protein structure coordinates were obtained from the PDB database and molecular graphics were performed with UCSF Chimera (15). When this information was absent for a protein of interest, we modeled the proteins according to their domains using available structures of similar domains to provide an approximate representation of the protein structure and size.