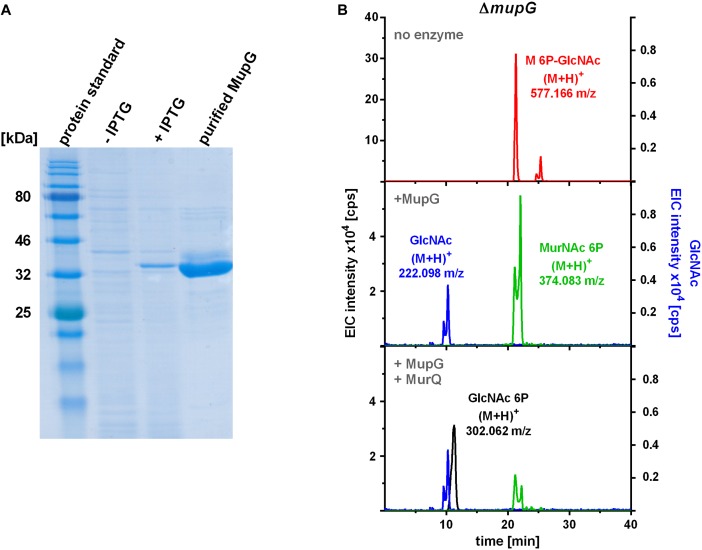
FIGURE 4.
Production of recombinant MupG and enzymatic digest of MurNAc 6P-GlcNAc extracted from ΔmupG cytosolic fractions. (A) MupG from S. aureus JE2 strain was heterologously expressed in E. coli BL21(DE3) cells. Protein expression, before (-IPTG) and after induction for 3 h (+IPTG), and purity (after purification by Ni2+-affinity and size exclusion chromatography) was controlled by SDS-PAGE (5 μg of the recombinant MupG protein was loaded). MupG-His6 protein has a calculated molecular weight of 41.5 kDa. (B) S. aureus ΔmupG cells were grown in LB medium for 24 h. Acetone extracts of the cytosolic fractions were analyzed by LC-MS in positive ion-mode. Fifty microliter of the cytosolic fraction from ΔmupG was incubated with buffer (no enzyme), with 1 μg recombinant MupG (+MupG) or with MupG and MurNAc 6P etherase MurQ (both 1 μg) for 2.5 h at 37°C. Representative mass spectra of the samples from at least three biological replicates were presented as extracted ion chromatograms (EIC × 104 [cps]) for MurNAc 6P-GlcNAc (observed masses of (M+H)+ = 577.166 m/z) in red, GlcNAc ((M+H)+ = 222.098 m/z) in blue, MurNAc 6P ((M+H)+ = 374.083 m/z) in green and GlcNAc 6P ((M+H)+ = 302.062) in black.