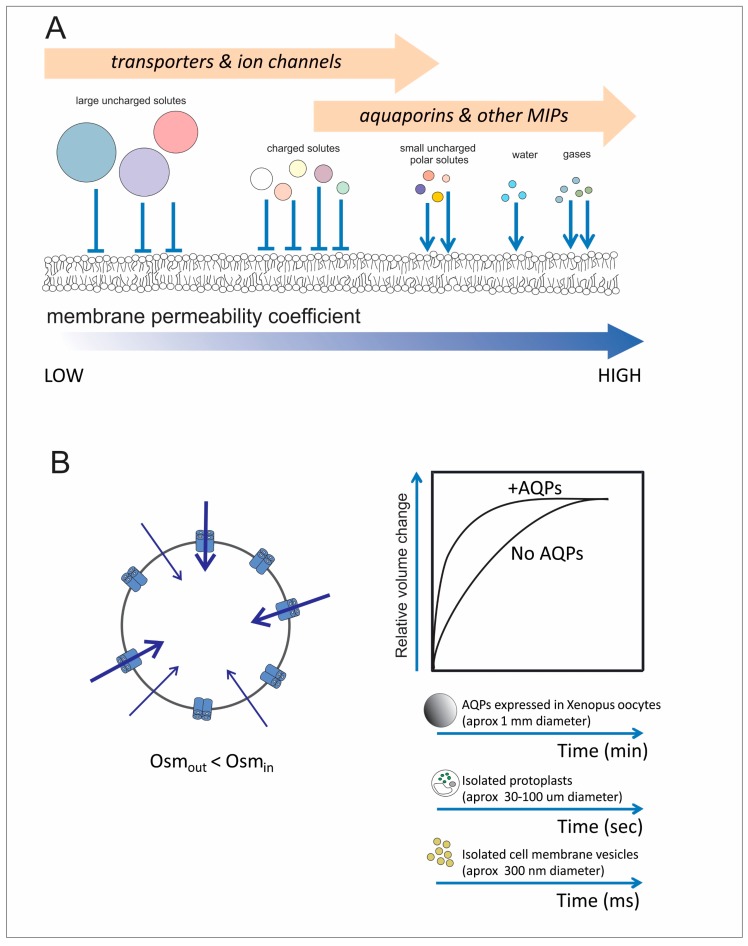
Figure 1.
Biological membranes and aquaporins. (A) Biological membranes are selectively permeable. Large solutes and polar ones (e.g., ions) have very low permeability coefficients and request specific protein transporters to facilitate their transfer. On the other hand, small uncharged polar solutes as well as water and gases have less resistance to permeate through the phospholipid bilayer and protein transporters are optional. However, now we know specific channels (aquaporins) are crucial molecular entities for controlling/regulating the rate of exchange of water, gases and certain solutes including ions in certain cases. In the scheme, the arrows represent the capacity to increase the membrane permeability by introducing integral membrane proteins into the phospholipid bilayer. We propose there is an overlap in the type of transporters that can be responsible for regulating the permeation pathway of a specific solute/water/gas. (B) Water exchange is facilitated when AQPs that are water channels are present. As the phospholipid bilayer is also permeable to water, osmotic swelling is possible under an imposed osmotic gradient even in the absence of aquaporins. However, their presence allows a faster swelling response. In the cartoon it is represented the water entry and the consequent cell swelling imposed by the osmotic gradient. Different techniques are available to measure water membrane permeability in isolated cells or smaller structures.