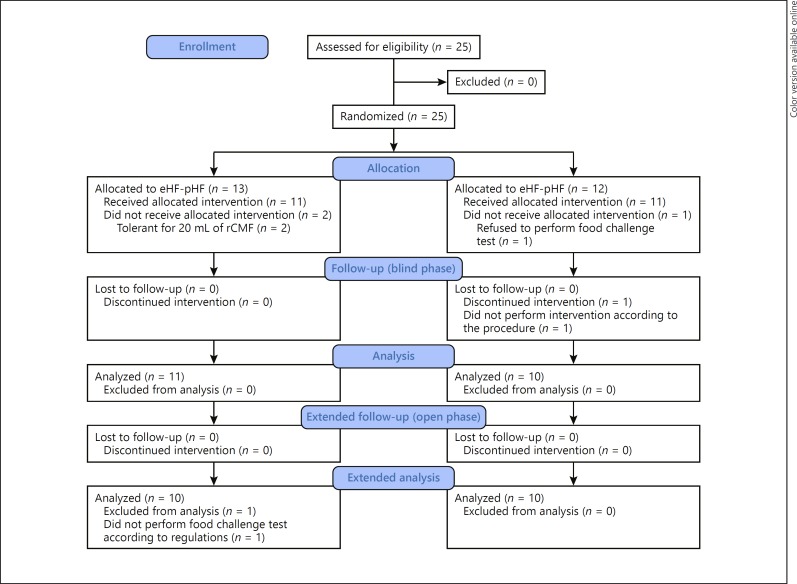
Fig. 2.
The number of participants assessed for eligibility for oral immunotherapy using partially hydrolyzed formula, who completed the trial. In total, 25 participants were enrolled and randomly assigned to 2 groups. At the baseline food challenge, 2 participants in the partially hydrolyzed cow's milk protein-based formula (pHF)-pHF group were tolerant to 20 mL of regular cow's milk formula (rCMF) and 1 in the extensively hydrolyzed cow's milk protein-based formula (eHF)-pHF group refused the food challenge test; these participants did not undergo the intervention. During the blind phase, 1 participant in the eHF-pHF group consumed his assigned formula mixed with other milk products during the first 2 weeks without our knowledge. When this became known, the participant was excluded from the trial. At the food challenge at the end of the open phase, 1 participant did not receive the food challenge formula according to the protocol; data for this participant were thus excluded from the analysis. The assignment of participants was blinded after all participants had undergone the intervention.