Fig. 3.
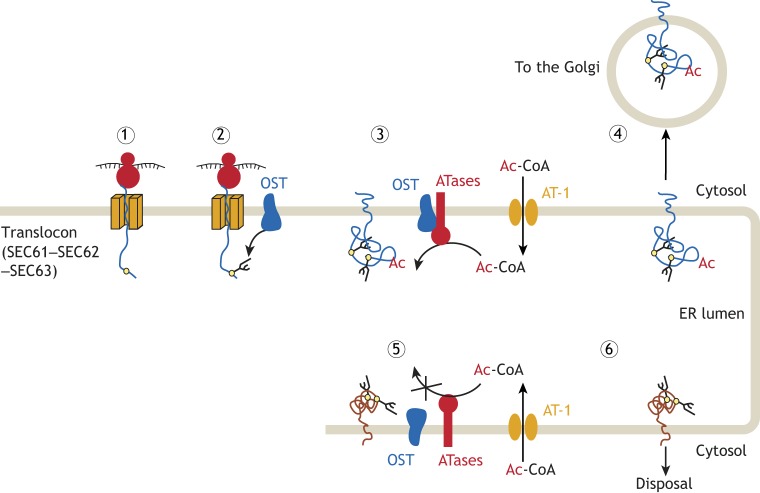
The ER acetylation machinery is an integral component of ER quality control and regulates the efficiency of the secretory pathway. Secretory membrane or lumenal proteins are synthesized close to the ER and enter the organelle through the translocon (SEC61–SEC62–SEC63) complex (1). Proteins with an N-x-S/T consensus sequence (yellow circles) for N-glycosylation that is 14 or 15 amino acids (∼40 Å) away from the inner membrane, are recognized by the oligosaccharyltransferase (OST) complex, which interacts with the translocon and transfers a preassembled GlcNAc2Man9Glc3 oligosaccharide (branched lines) to the asparagine residue of the nascent glycoprotein (2). Correctly folded glycoproteins are then recognized by the ATases, which interact with the OST to acetylate specific lysine residues (3). The acetylated lysine residues act as a positive marker that allows correctly folded glycoproteins to advance toward the Golgi (4). Unfolded or misfolded glycoproteins, although N-glycosylated, are not recognized by the ATases (5), and, as a result, are prevented from reaching the Golgi and are disposed of (6). Although supported by a number of publications (see text), the above is only a working model for how the ER acetylation machinery might regulate the secretory pathway.