Fig. 8.
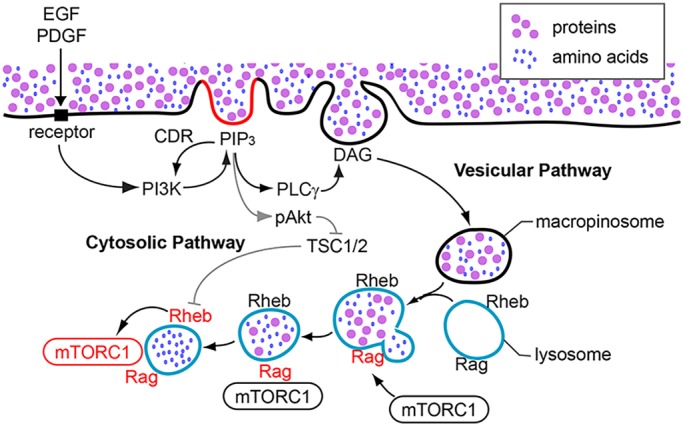
A model for CDR-dependent Akt phosphorylation and activation of mTORC1. EGF and low concentrations of PDGF induce mTORC1 activation via cytosolic and vesicular pathways, both of which are regulated by CDRs. After stimulation, CDRs are formed by cytoskeletal activities and activated PI3K generates PIP3 at the plasma membrane. The molecular mechanism of PIP3 restriction to CDRs is still unknown, but could be caused by positive feedback amplification of PI3K by PIP3, supported by diffusion barriers in CDRs. Elevated concentrations of PIP3 inside CDRs recruit Akt to the plasma membrane (cytosolic pathway). Akt localized to the plasma membrane is phosphorylated on threonine 308 and serine 473 by PDK1 and mTORC2, respectively. pAkt then phosphorylates TSC2, resulting in the loss of GAP function of the TSC1/2 complex towards Rheb. Rheb is then activated at the lysosomes. Meanwhile, PIP3 at CDRs also activates PLCγ, which generates diacylglycerol (DAG) in CDRs, leading to the formation of macropinosomes (vesicular pathway). Macropinosomes convey extracellular nutrients into lysosomes, which induce the activation of Rag. Rag recruits mTORC1 to the lysosomes, where mTORC1 is activated by Rheb. This study supports a role for CDRs in amplification of PI3K and pAkt.
