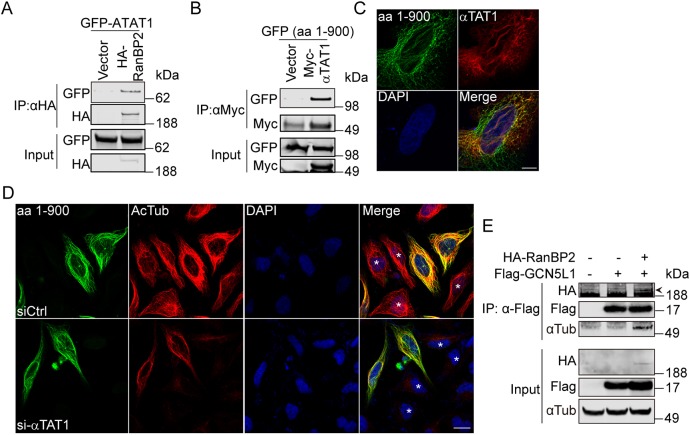
Fig. 7.
RanBP2 recruits the GCN5L1–αTAT1 complex to facilitate α-tubulin acetylation. (A) RanBP2 co-immunoprecipitated with αTAT1. 293T cells were transfected with the indicated expression plasmids, then anti-HA immunoprecipitates were subjected to anti-HA and anti-GFP immunoblot analysis. (B) The N-terminal region of RanBP2 was required for the association with αTAT1. The GFP-tagged N-terminal fragment of RanBP2 [amino acids (aa) 1–900] was expressed in 293T cells with control vector or Myc–αTAT1 and the cells lysates were subjected to immunoprecipitate using anti-Myc antibody. The immunoprecipitates were probed with anti-GFP and anti-Myc antibody. (C) Confocal microscopy showed colocalization of RanBP2 (aa 1–900) with αTAT1. HeLa cells were co-transfected with GFP-tagged N-terminal RanBp2 (aa 1–900) (green) and Flag–αTAT1 (red); then the localization was analyzed with anti-Flag antibodies. GFP fluorescence was visualized directly. DNA was visualized by DAPI staining (blue). Scale bar: 10 µm. (D) Knockdown αTAT1 abolished the RanBP2-induced α-tubulin acetylation (Ac-Tub). GFP-tagged N-terminal RanBp2 (aa 1–900)-transfected 293T cells were mock-transfected (siCtrl) or transfected with αTAT1 siRNA (si-αTAT1) and the Ac-Tub levels (red) were visualized by immunostaining. Non-transfected cells are marked with an asterisk (*) in the merge panels. Scale bar: 20 µm. (E) RanBP2 recruited GCN5L1 to microtubules. 293T cells were transfected with indicated expression vectors, then the anti-Flag immunoprecipitates were subjected to anti-GFP, anti-Flag and anti-α-tubulin immunoblot analysis. The specific band for HA–RanBP2 (depicted with an arrowhead) was evident at a higher molecular mass than a non-specific band. Representative immunoblots of three independent experiments.