Abstract
Background
Public health strategies that target mosquito vectors, particularly pyrethroid long‐lasting insecticidal nets (LLINs), have been largely responsible for the substantial reduction in the number of people in Africa developing malaria. The spread of insecticide resistance in Anopheles mosquitoes threatens these impacts. One way to control insecticide‐resistant populations is by using insecticide synergists. Piperonyl butoxide (PBO) is a synergist that inhibits specific metabolic enzymes within mosquitoes and has been incorporated into pyrethroid‐LLINs to form pyrethroid‐PBO nets. Pyrethroid‐PBO nets are currently produced by four LLIN manufacturers and, following a recommendation from the World Health Organization (WHO) in 2017, are being included in distribution campaigns in countries. This review examines epidemiological and entomological evidence on whether the addition of PBO to LLINs improves their efficacy.
Objectives
1. Evaluate whether adding PBO to pyrethroid LLINs increases the epidemiological and entomological effectiveness of the nets.
2. Compare the effects of pyrethroid‐PBO nets currently in commercial development or on the market with their non‐PBO equivalent in relation to:
a. malaria infection (prevalence or incidence); b. entomological outcomes.
Search methods
We searched the Cochrane Infectious Diseases Group (CIDG) Specialized Register; CENTRAL, MEDLINE, Embase, Web of Science, CAB Abstracts, and two clinical trial registers (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform) up to 24 August 2018. We contacted organizations for unpublished data. We checked the reference lists of trials identified by the above methods.
Selection criteria
We included laboratory trials, experimental hut trials, village trials, and randomized clinical trials with mosquitoes from the Anopheles gambiae complex or Anopheles funestus group.
Data collection and analysis
Two review authors assessed each trial for eligibility, extracted data, and determined the risk of bias for included trials. We resolved disagreements through discussion with a third review author. We analysed the data using Review Manager 5 and assessed the certainty of the evidence using the GRADE approach.
Main results
Fifteen trials met the inclusion criteria: two laboratory trials, eight experimental hut trials, and five cluster‐randomized controlled village trials.
One village trial examined the effect of pyrethroid‐PBO nets on malaria infection prevalence in an area with highly pyrethroid‐resistant mosquitoes. The latest endpoint at 21 months post‐intervention showed that malaria prevalence probably decreased in the intervention arm (OR 0.40, 95% CI 0.20 to 0.80; 1 trial, 1 comparison, moderate‐certainty evidence).
In highly pyrethroid‐resistant areas (< 30% mosquito mortality), in comparisons of unwashed pyrethroid‐PBO nets to unwashed standard‐LLINs, PBO nets resulted in higher mosquito mortality (risk ratio (RR) 1.84, 95% CI 1.60 to 2.11; 14,620 mosquitoes, 5 trials, 9 comparisons, high‐certainty evidence) and lower blood feeding success (RR 0.60, 95% CI 0.50 to 0.71; 14,000 mosquitoes, 4 trials, 8 comparisons, high‐certainty evidence). However, in comparisons of washed pyrethroid‐PBO nets to washed LLINs we do not know if PBO nets have a greater effect on mosquito mortality (RR 1.20, 95% CI 0.88 to 1.63; 10,268 mosquitoes, 4 trials, 5 comparisons, very low‐certainty evidence), although the washed pyrethroid‐PBO nets do decrease blood feeding success compared to standard‐LLINs (RR 0.81, 95% CI 0.72 to 0.92; 9674 mosquitoes, 3 trials, 4 comparisons, high‐certainty evidence).
In areas where pyrethroid resistance is considered moderate (31% to 60% mosquito mortality), there may be little or no difference in effects of unwashed pyrethroid‐PBO nets compared to unwashed standard‐LLINs on mosquito mortality (RR 1.16, 95% CI 0.88 to 1.54; 242 mosquitoes, 1 trial, 1 comparison, low‐certainty evidence), and there may be little or no difference in the effects on blood feeding success (RR 0.87, 95% CI 0.67 to 1.13; 242 mosquitoes, 1 trial, 1 comparison, low‐certainty evidence). The same pattern is apparent for washed pyrethroid‐PBO nets compared to washed standard‐LLINs (mortality: RR 1.07, 95% CI 0.74 to 1.54; 329 mosquitoes, 1 trial, 1 comparison, low‐certainty evidence; blood feeding success: RR 0.91, 95% CI 0.74 to 1.13; 329 mosquitoes, 1 trial, 1 comparison, low‐certainty evidence).
In areas where pyrethroid resistance is low (61% to 90% mosquito mortality), there is probably little or no difference in the effect of unwashed pyrethroid‐PBO nets compared to unwashed standard‐LLINs on mosquito mortality (RR 1.10, 95% CI 1.05 to 1.16; 708 mosquitoes, 1 trial, 2 comparisons, moderate‐certainty evidence), but there is no evidence for an effect on blood feeding success (RR 0.67, 95% CI 0.06 to 7.37; 708 mosquitoes, 1 trial, 2 comparisons, very low‐certainty evidence). For washed pyrethroid‐PBO nets compared to washed standard‐LLINs we do not know if there is any difference in mosquito mortality (RR 1.16, 96% CI 0.83 to 1.63; 878 mosquitoes, 1 trial, 2 comparisons, very low‐certainty evidence), but blood feeding may decrease (RR 1.50, 95% CI 0.89 to 2.54; 878 mosquitoes, 1 trial, 2 comparisons, low‐certainty evidence).
In areas were mosquito populations are susceptible to insecticides (> 90% mosquito mortality), there may be little or no difference in the effect of unwashed pyrethroid‐PBO nets compared to unwashed standard‐LLINs on mosquito mortality (RR 1.20, 95% CI 0.64 to 2.26; 2791 mosquitoes, 2 trials, 2 comparisons, low‐certainty evidence). This is similar for washed nets (RR 1.07, 95% CI 0.92 to 1.25; 2644 mosquitoes, 2 trials, 2 comparisons, low‐certainty evidence). We do not know if unwashed pyrethroid‐PBO nets have any effect on blood feeding success of susceptible mosquitoes (RR 0.50, 95% CI 0.11 to 2.32; 2791 mosquitoes, 2 trials, 2 comparisons, very low‐certainty evidence). The same applies to washed nets (RR 1.28, 95% CI 0.81 to 2.04; 2644 mosquitoes, 2 trials, 2 comparisons, low‐certainty evidence).
In village trials comparing pyrethroid‐PBO nets to LLINs, there was no difference in sporozoite rate (4 trials, 5 comparison) and mosquito parity (3 trials, 4 comparisons).
Authors' conclusions
In areas of high insecticide resistance, pyrethroid‐PBO nets increase mosquito mortality and reduce blood feeding rates, and results from a single clinical trial demonstrate that this leads to lower malaria prevalence. Questions remain about the durability of PBO on nets, as the impact of pyrethroid‐PBO LLINs on mosquito mortality was not sustained over 20 washes in experimental hut trials. There is little evidence to support higher entomological efficacy of pyrethroid‐PBO nets in areas where the mosquitoes show lower levels of resistance to pyrethroids.
17 September 2019
Up to date
All studies incorporated from most recent search
All published trials found in the last search (24 Aug, 2018) were included, and we identified two ongoing studies
Plain language summary
Pyrethroid‐PBO nets to prevent malaria
Background
Bed nets treated with pyrethroid insecticides are an effective way to reduce malaria transmission and have been deployed across Africa. However, mosquitoes that spread malaria are now developing resistance to this type of insecticide. One way to overcome this resistance is to add another chemical, piperonyl butoxide (PBO), to the net. PBO is not an insecticide but blocks the substance (an enzyme) inside the mosquito that stops pyrethroids working.
What is the aim of this review?
The aim of this Cochrane Review was to find out if pyrethroid‐PBO nets add additional protection against malaria when compared to standard pyrethroid‐only nets.
Key messages
Pyrethroid‐PBO nets are more effective than standard pyrethroid‐only nets in killing mosquitoes and preventing them from blood feeding in areas where the mosquito populations are very resistant to pyrethroid insecticides (high‐certainty evidence). Pyrethroid‐PBO nets probably reduce the number of malaria infections (moderate‐certainty evidence), although further high‐quality studies measuring clinical outcomes are needed.
What was studied in the review?
We included 15 trials conducted between 2010 to 2018 that compared standard pyrethroid nets to pyrethroid‐PBO nets. These consisted of two laboratory trials, eight experimental hut trials that measured the impact of the pyrethroid‐PBO nets on a wild population of mosquitoes, and five village trials. Only one village trial measured the impact of pyrethroid‐PBO nets on malaria infection in humans; all other studies recorded the impact on mosquito populations. We analysed all studies to determine whether the pyrethroid‐PBO nets were better at killing mosquitoes and preventing them from blood feeding. For the single clinical trial, we examined whether pyrethroid‐PBO nets reduced the number of malaria infections. As the benefit of adding PBO to nets is likely to depend on the level of pyrethroid resistance in the mosquito population, we performed separate analyses for studies conducted in areas of high‐, medium‐, and low‐levels of pyrethroid resistance.
What are the main results of the review?
Where mosquitoes show high levels of resistance to pyrethroids, pyrethroid‐PBO nets perform better than standard pyrethroid‐only nets at killing mosquitoes and preventing them from blood feeding. As expected, this effect is not seen in areas where the mosquitoes show low or no resistance to the pyrethroid‐only insecticides. Only one trial looked at the impact of using pyrethroid‐PBO nets on the number of people infected with the malaria parasite. This trial, involving 3966 participants and conducted in an area where mosquitoes are very resistant to pyrethroids, found that fewer people were infected with malaria when the population used pyrethroid‐PBO nets compared to standard pyrethroid‐only nets.
How up to date is this review?
We searched for studies that had been published up to 24 August 2018.
Summary of findings
Background
Description of the condition
Substantial progress has been made in reducing the burden of malaria in the 21st century. It is estimated that the clinical incidence of Plasmodium falciparum malaria in Africa dropped by 40% between 2000 and 2015, equating to the prevention of 663 million cases (Bhatt 2015; WHO‐GMP 2015). However progress has stalled in recent years (WHO 2017a). Targeting the mosquito vector has proven to be the most effective method of malaria prevention in Africa, with over two‐thirds of the malaria cases averted in the first 15 years of this century attributed to scale‐up in the use of long‐lasting insecticidal nets (LLINs), (Bhatt 2015). This method of malaria prevention is particularly effective in Africa where the major malaria vectors, Anopheles gambiae and Anopheles funestus, are largely endophagic (feed indoors) and endophilic (rest indoors after blood feeding).
Currently only one insecticide class, the pyrethroids, is commonly used to treat LLINs; pyrethroids have the required dual properties of low mammalian toxicity and rapid insecticidal activity (Zaim 2000), and their repellent or contact irritant effects may enhance the personal protection of LLINs. Unfortunately, resistance to pyrethroids is now widespread in African malaria vectors (Ranson 2016). This may be the result of mutations in target‐site proteins (target‐site resistance), (Ranson 2011; Ridl 2008), which results in reduced sensitivity to the insecticide or increased activity of detoxification enzymes (metabolic resistance) (Mitchell 2012; Stevenson 2011), or other as yet poorly‐described resistance mechanisms, or a combination of all or some of these factors. The evolution of insecticide resistance and its continuing spread threatens the operational success of malaria vector control interventions. The current impact of this resistance on malaria transmission is largely unquantified and will vary depending on the level of resistance, malaria endemicity, and proportion of the human population using LLINs (Churcher 2016). A recent multi‐country trial found no evidence that pyrethroid resistance reduced the personal protection provided by use of LLINs (Kleinschmidt 2018). However, it is generally accepted that resistance will eventually erode the efficacy of pyrethroid‐only LLINs and that innovation in the LLIN market is essential to maintain the efficacy of this preventative measure (MPAC 2016).
Description of the intervention
One way of controlling insecticide‐resistant mosquito populations is through the use of insecticide synergists. Synergists are generally non‐toxic and act by enhancing the potency of insecticides. Piperonyl butoxide (PBO) is a synergist that inhibits specific metabolic enzymes within mosquitoes, and has been incorporated into pyrethroid‐treated LLINs to form PBO‐combination nets (hereafter referred to as pyrethroid‐PBO nets). Insecticide‐synergist combination nets represent a new product class with the capacity to affect insecticide‐resistant populations. In 2017 the World Health Organization (WHO), gave pyrethroid‐PBO nets an interim endorsement as a new vector control class and recommended that countries consider deploying these nets in areas where pyrethroid resistance has been confirmed in the main malaria vectors (WHO‐GMP 2017a).
Currently there are five pyrethroid‐PBO nets in production: Olyset® Plus; PermaNet® 3.0; Veeralin® LN; and DawaPlus 3.0 and 4.0. Olyset Plus, which is manufactured by Sumitomo Chemical Company Ltd, is a polyethylene net treated with permethrin (20 g/kg ± 25%) and PBO (10 g/kg ± 25%) across the whole net (Sumitomo 2013). PermaNet 3.0, which is manufactured by Vestergaard Frandsen, is a mixed polyester (sides) polyethylene (roof) net treated with deltamethrin and PBO; PBO is only found on the roof of the net (25 g/kg ± 25%) and the concentration of deltamethrin varies depending on location (roof: 4.0 g/kg ± 25%) and yarn type (sides: 75 denier (thickness) yarn with 70 cm lower border 2.8 g/kg ± 25%, 100 denier (thickness) yarn without border 2.1 g/kg ± 25%; Vestergaard 2015). Veeralin LN, manufactured by Vector Control Innovations Private Ltd, is a polyethylene net treated with alpha‐cypermethrin (6.0 g/kg) and PBO (2.2 g/kg) across the whole net (WHOPES 2016). DawaPlus 3.0 and 4.0 are manufactured by Tana Netting, UAE, and contain PBO on the roof only (3.0), or on all sides (4.0); deltamethrin suspension concentrate (SC) is coated on knitted multi‐filament polyester fibres, at the target dose of 1.33 g/kg in 75 denier (thickness) yarn and 1 g/kg in 100 denier (thickness) yarn, corresponding to 40 mg of deltamethrin per m2, using a polymer as a binder. Ownership of DawaPlus 4.0 is currently in the process of transferring ownership to NRS Moon netting FZE (WHO 2018).
How the intervention might work
PBO inhibits metabolic enzyme families, in particular the cytochrome P450 enzymes that detoxify or sequester pyrethroids. Increased production of P450s is thought to be the most potent mechanism of pyrethroid resistance in malaria vectors, and pre‐exposure to PBO has been shown to restore susceptibility to pyrethroids in laboratory bioassays on multiple pyrethroid‐resistant vector populations (Churcher 2016).
Widespread use of conventional LLINs provides both personal and community protection from malaria (Bhatt 2015; Lengeler 2004). In areas where the mosquito populations are resistant to pyrethroids, experimental hut trials (as described in the ‘Types of studies' section), have shown that mosquito mortality rates and protection from blood feeding are substantially reduced when using conventional LLINs (Abílio 2015; Awolola 2014; Bobanga 2013; N'Guessan 2007; Riveron 2015; Yewhalaw 2012). The addition of PBO to pyrethroids in LLINs can restore the killing effects of LLINs in areas where this has been eroded by insecticide resistance. LLINs that contain PBO have been evaluated in multiple experimental hut trials across Africa (Adeogun 2012; Corbel 2010; Koudou 2011; N'Guessan 2010; Pennetier 2013; Tungu 2010). In most settings, the pyrethroid‐PBO nets resulted in higher rates of mosquito mortality and greater blood‐feeding inhibition than conventional LLINs, although the magnitude of this effect was variable. Village trials have measured the impact on sporozoite infection rates in mosquitoes with mixed results (Awolola 2014; Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013). Recently, a cluster‐randomized trial in Tanzania demonstrated that use of pyrethroid‐PBO nets can reduce malaria prevalence in children (Protopopoff 2018).
Why it is important to do this review
All LLINs approved by the WHO Prequalification Team (formerly the WHO Pesticide Evaluation Scheme (WHOPES)), contain pyrethroids as the sole active ingredient. Five LLINs that contain PBO have interim approval (WHOPES 2016), and have been recognized as a new product class by WHO (WHO‐GMP 2017a). As pyrethroid‐PBO nets are generally more expensive than conventional LLINs, it is important to determine if they are superior to conventional LLINs and, if so, under what circumstances, to enable cost‐effectiveness trials to be performed to inform procurement decisions.
An Expert Review Group (ERG) commissioned by the WHO has recommended pyrethroid‐PBO nets be considered for use in areas where the major malaria vectors are resistant to pyrethroids (WHO‐GMP 2017a). This recommendation was largely based on a single randomized controlled trial of one pyrethroid‐PBO net type conducted in Tanzania (Protopopoff 2018), but also supported by a meta‐analysis of performance of pyrethroid‐PBO nets in experimental hut trials, which was used to parameterize a malaria transmission model to predict the public health benefit of pyrethroid‐PBO nets (Churcher 2016). However confusion remains over the settings in which pyrethroid‐PBO nets should be deployed. The WHO recommendation is that countries should consider deployment of this new product class in areas with intermediate levels of pyrethroid resistance but calls for further evidence, including data from a second clinical trial. This guidance has been adopted by some net providers; for example, the President's Malaria Initiative (PMI) (PMI 2018).
In an attempt to assess the evidence of the effectiveness of pyrethroid‐PBO nets against African malaria vectors in areas with differing levels of insecticide resistance, we have conducted a systematic review of all relevant trials and examined both epidemiological and entomological endpoints. We appreciate that the evaluation of PBO will depend on trials where the background insecticide and dose is the same in both intervention and control groups and are aware that most trials have evaluated pyrethroid‐PBO nets against pyrethroid‐only LLINs with different background insecticides and doses, which confounds the effects.
One of the problems with this research field is that pyrethroid‐PBO nets are commercial products. The pyrethroid‐PBO nets currently undergoing clinical trials have had additional alterations made to them, such as changing the concentration or rate at which the pyrethroid is released. However, these are the products for which policy decisions are needed, based on evidence related to their relative effectiveness. Thus, in this Cochrane Review, we sought direct evidence for PBO alone on the effectiveness of nets, and examined the evidence concerning the effectiveness of commercial products. During these comparisons, we considered other potential confounding factors.
Objectives
Evaluate whether adding PBO to pyrethroid LLINs increases the epidemiological and entomological effectiveness of the nets.
-
Compare the effects of pyrethroid‐PBO nets currently in commercial development or on the market with their non‐PBO equivalent in relation to:
malaria infection (prevalence or incidence);
entomological outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Objective 1
We included:
laboratory bioassay trials (for example, cone bioassays, tunnel tests);
experimental hut trials; and
village trials.
Objective 2 (a and b)
We included:
experimental hut trials;
village trials; and
clinical trials.
See Table 5 for WHOPES definitions in detail.
1. World Health Organization Pesticide Evaluation Scheme (WHOPES) classification.
| WHOPES Phase | Definition |
| WHOPES Phase I. Laboratory bioassays | Cone bioassays: these are studies that are conducted in the laboratory setting and use standard WHO protocols (WHO 2013, Section 2.2.1), where mosquitoes are exposed to a suitable LLIN (treated intervention or untreated control), for three minutes using a standard plastic WHO cone. Following net exposure, mosquitoes are transferred to a holding container and maintained on a sugar solution diet while entomological outcomes (mosquitoes knocked down one hour post‐exposure, and mosquito mortality 24 hours post‐exposure), are measured. Tunnel tests: these are studies conducted in the laboratory setting that use standard WHO protocols (WHO 2013, Section 2.2.2). Mosquitoes are released into a glass tunnel covered at each end with untreated netting. The intervention or control LLIN net sample is placed one‐third down the length of the tunnel and the net contains nine holes that enable mosquitoes to pass through. A suitable bait is immobilized in the shorter section of the tunnel where it is available for mosquito biting. Mosquitoes are released into the opposite end of the tunnel and must make contact with the net and locate holes before being able to feed on the bait. After 12 to 15 hours, mosquitoes are removed from both sections of the tunnel and entomological outcomes (the number of mosquitoes in each section, mortality, and blood‐feeding inhibition at the end of the assay and 24 hours post‐exposure), are recorded. Wire‐ball bioassays: these are studies conducted in the laboratory setting where mosquitoes are introduced into a wire‐ball frame that has been covered with either the intervention or control LLIN. Mosquitoes are exposed for three minutes, after which they are transferred to a holding container and entomological outcomes (mosquitoes knocked down one hour post‐exposure, and mosquito mortality 24 hours post‐exposure), are measured. |
| WHOPES Phase II. Experimental hut trials | WHOPES Phase II experimental hut trials are field trials conducted in Africa where wild mosquito populations or local colonized populations are evaluated. Volunteers or livestock sleep in experimental huts under a purposefully holed LLIN, with one person or animal per hut. Huts are designed to resemble local housing based on a West or East African design (WHO 2013; Section 3.3.1‐2). However they have identical design features, such as eave gaps or entry slits to allow mosquitoes to enter, and exit traps to capture exiting mosquitoes. LLINs and volunteers are randomly allocated to huts and rotated in a Latin square to avoid bias, with huts cleaned between rotations to avoid contamination. Several nets, including an untreated control net, can be tested at the same time. Dead and live mosquitoes are collected each morning from inside the net, inside the hut, and inside the exit traps. They are then scored as either blood‐fed or non‐blood‐fed, and either alive or dead, and live mosquitoes are maintained for a further 24 hours to assess delayed mosquito mortality. |
| WHOPES Phase III. Village trials | WHOPES Phase III village trials are village trials conducted in Africa where wild mosquito populations are evaluated. Villages chosen to be included in the study are similar in terms of size, housing structure, location, and the data available on the insecticide resistance status of the local malaria vectors. Households are assigned either conventional LLINs or PBO‐LLINs. Randomization can be at the household or village level. Adult mosquitoes are collected from the study houses and mosquito density is measured. An indication of malaria transmission is measured in the study sites either by recording infections in mosquitoes, malaria prevalence, or malaria incidence. |
Abbreviations: LLIN: long‐lasting insecticidal nets; PBO: pyrethroid‐piperonyl butoxide; WHOPES: World Health Organization Pesticide Evaluation Scheme
Types of participants
Mosquitoes
Anopheles gambiae complex or Anopheles funestus group. Included trials had to test a minimum of 50 mosquitoes per trial arm. We included all mosquito populations and examined the insecticide resistance level (measured using phenotypic resistance) during data analysis.
Humans
Adults and children living in malaria‐endemic areas.
Types of interventions
Intervention
Combination LLIN treated with both PBO and a pyrethroid insecticide. The LLINs must have received a minimum of interim‐WHO approval (Table 6), and LLINs had to be treated with a WHO‐recommended dose of pyrethroid (Table 7).
2. World Health Organization (WHO)‐recommended long‐lasting insecticidal nets (LLINs).
| Product name | Product type | Status of WHO recommendation |
| DawaPlus 2.0 | Deltamethrin coated on polyester | Interim |
| DawaPlus 3.0 | Combination of deltamethrin coated onto polyester (side panels), and deltamethrin and PBO incorporated into polyester (roof) | Interim |
| DawaPlus 4.0 | Deltamethrin and PBO incorporated into polyester | Interim |
| Duranet | Alpha‐cypermethrin incorporated into polyethylene | Full |
| Interceptor | Alpha‐cypermethrin coated on polyester | Full |
| Interceptor G2 | Alpha‐cypermethrin and chlorfenapyr incorporated into polyester | Interim |
| LifeNet | Deltamethrin incorporated into polypropylene | Interim |
| MAGNet | Alpha‐cypermethrin incorporated into polyethylene | Full |
| MiraNet | Alpha‐cypermethrin incorporated into polyethylene | Interim |
| Olyset Net | Permethrin incorporated into polyethylene | Full |
| Olyset Plus | Permethrin (20g/kg) and PBO (10g/kg) incorporated into polyethylene | Interim |
| Panda Net 2.0 | Deltamethrin incorporated into polyethylene | Interim |
| PermaNet 2.0 | Deltamethrin coated on polyester | Full |
| PermaNet 3.0 | Combination of deltamethrin coated on polyester with strengthened border (side panels), and deltamethrin and PBO incorporated into polyethylene (roof) | Interim |
| Royal Sentry | Alpha‐cypermethrin incorporated into polyethylene | Full |
| SafeNet | Alpha‐cypermethrin coated on polyester | Full |
| Veeralin | Alpha‐cypermethrin and PBO incorporated into polyethylene | Interim |
| Yahe | Deltamethrin coated on polyester | Interim |
| Yorkool | Deltamethrin coated on polyester | Full |
Abbreviations: LLIN: long‐lasting insecticidal nets; PBO: pyrethroid‐piperonyl butoxide; WHO: World Health Organization.
3. World Health Organization (WHO)‐recommended insecticide products for treatment of mosquito nets for malaria vector control.
| Insecticide | Formulation | Dosagea |
| Alpha‐cypermethrin | SC 10% | 20‐40 |
| Cyfluthrin | EW 5% | 50 |
| Deltamethrin | SC 1% WT 25% WT 25% + binderb | 15‐25 |
| Etofenprox | EW 10% | 200 |
| Lambda‐cyhalothrin | CS 2.5% | 10‐15 |
| Permethrin | EC 10% | 200‐500 |
Abbreviations: EC: emulsifiable concentrate; EW: emulsion, oil in water; CS: capsule suspension; SC: suspension concentrate; WT: water dispersible tablet. aActive ingredient/netting (mg/m2). bK‐O TAB 1‐2‐3.
Control
Conventional LLINs that contain pyrethroid only.
For objective 1 nets of the same fabric had to be treated with the same insecticide, dose and release rate as the intervention net to allow direct evaluation of the addition of PBO.
For objective 2 (a and b), nets could be treated with the same insecticide at different doses from the intervention net to allow critical appraisal of all pyrethroid‐PBO nets currently in development or on the market.
For both intervention and control arms, nets could be unholed, holed, unwashed or washed, providing the trials adhered to WHO guidelines (WHO 2013).
Types of outcome measures
Trials had to include at least one of the following outcomes to be eligible for inclusion: mosquito knock‐down, mosquito mortality, blood feeding, sporozoite rate, parasite presence, clinical malaria confirmation, not passed through net, deterrence, exophily, mosquito density, or parity rate.
Primary outcomes
Epidemiological
Parasite presence: presence of malaria parasites through microscopy of blood or Rapid Diagnostic Tests (RDTs).
Clinical malaria confirmation: clinical diagnosis based on the participant's symptoms and on physical findings at examination.
Entomological
Mosquito mortality: immediate death or delayed death (up to 24 hours), or both, measured as a proportion of total mosquito number. A mosquito is classified as dead if it is immobile, cannot stand or fly, or shows no sign of life.
Mosquito knock‐down: mosquito ‘mortality' recorded one hour post‐insecticide exposure, termed ‘knock‐down' as some mosquitoes may recover during the 24‐hour recovery period before mosquito mortality is recorded at 24 hours post‐exposure.
Blood‐feeding success: number of mosquitoes that have blood‐fed (alive or dead).
Sporozoite rate: percentage of mosquitoes with sporozoites in salivary glands.
Secondary outcomes
Entomological
Not passed through net: the number of mosquitoes that do not pass through a holed pyrethroid‐PBO net to reach a human bait relative to a standard LLIN, using a tunnel test.
Deterrence: the number of mosquitoes that enter a hut that is using a pyrethroid‐PBO net relative to the number of mosquitoes found in a control hut that is using a standard LLIN (experimental hut trials only).
Exophily: the proportion of mosquitoes found in exit/veranda traps of a hut that is using a pyrethroid‐PBO net, relative to the control hut that is using a standard LLIN (experimental hut trials only).
Mosquito density: measured using all standard methods, such as window exit traps, indoor resting collections, floor sheet collections, pyrethrum spray catch, and light traps (village trials).
Parity rate: percentage of parous mosquitoes which are detected by mosquito ovary dissections (village trials).
Search methods for identification of studies
We identified all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress). We have presented the MEDLINE search strategy in Appendix 1.
Electronic searches
Vittoria Lutje, the Cochrane Infectious Diseases Group (CIDG) Information Specialist, searched the following databases on 24 August 2018 using the search terms and strategy described in Appendix 1: the CIDG Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL, included in the Cochrane Library (Issue 8 2018); MEDLINE (PubMed); Embase (OVID); Web of Science Core Collection, and CAB Abstracts. She also searched the WHO International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov/ct2/home) for trials in progress.
Searching other resources
We contacted the following organizations for unpublished data: the PMI; the Innovative Vector Control Consortium (IVCC); Vestergaard Frandsen; Sumitomo Chemical Company Ltd; Vector Control Innovations Private Ltd; Endura SpA; and WHOPES. We checked the reference lists of trials identified by the above methods.
Data collection and analysis
All analyses were stratified by trial design. We also performed analyses for the primary outcomes stratified by both trial design and mosquito insecticide resistance level where possible. In addition, for hut trials conducted in high‐resistance areas, we also performed analyses stratified by net type.
We determined whether mosquito populations are susceptible or resistant to pyrethroid insecticides based on WHO definitions (WHO 2016; Table 8). We used 24‐hour mosquito mortality to determine resistance status, however if this had been unavailable, we intended to use knock‐down 60 minutes after the end of the assay. We stratified resistant populations into low‐, moderate‐, or high‐prevalence resistance (Table 9), by dividing the resistant mosquitoes (that is, those with less than 90% mortality) into three equal groups, with the lower third being the most resistant and upper third the most susceptible.
4. Definition of resistance level.
| Outcome | Confirmed resistance | Suspected resistance | Susceptible | Unclassified |
| WHO mosquito mortalitya | < 90% | 90% to 97% | 98% to 100% | Unknown |
| CDC knock‐downb | < 90% | 80% to 97% | 98% to 100% | Unknown |
Abbreviations: CDC: Centers for Disease Control and Prevention; WHO: World Health Organization. aDefinition of resistance level based on mosquito mortality (%) after exposure to insecticide in a WHO diagnostic dose assay. bDefinition of resistance level based on mosquito mortality (%) after exposure to insecticide in a CDC bottle bioassay using the methodology, diagnostic doses, and diagnostic times recommended by each test respectively.
5. Stratification of resistance level.
| Outcome | Low | Moderate | High | Unclassified |
| Mosquito mortalitya | 61% to 90% | 31% to 60% | < 30% | Unknown |
a24‐hour post‐exposure mortality (%).
Selection of studies
Two review authors (KG and NL) independently screened titles and abstracts of all retrieved references based on the inclusion criteria (Table 10). We resolved any inconsistencies between the review authors' selections by discussion. If we were unable to reach an agreement, we consulted a third review author (HR). We retrieved the full‐text trial reports for all potentially relevant citations. Two review authors independently screened the full‐text articles and identified trials for inclusion, and identified and recorded reasons for exclusion of ineligible trials in a ‘Characteristics of excluded studies' table. We resolved any disagreements through discussion or, if required, consulted a third review author (HR). We identified and excluded duplicates and collated multiple reports of the same trial so that each trial, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
6. Study inclusion screening form.
| Criteria | Assessment | Comments | ||
| Yes | No | Unclear | ||
| Mosquito population | ||||
| Did the study test Anopheles gambiae complex or Anopheles funestus group mosquitoes? | ↓ | — | ↓ | State mosquito species |
| Were a minimum of 50 mosquitoes tested per study arm? | ↓ | — | ↓ | |
| Intervention | ||||
| Did the study include an long‐lasting insecticidal net (LLIN) or insecticide‐treated net (ITN)? | ↓ | — | ↓ | State net LLIN or ITN |
Was the intervention net either of the following?
|
↓ | — | ↓ | State net type |
Was the control net either of the following?
|
↓ | — | ↓ | State which objective study meets |
| Study design | ||||
Was the study one of the following?
|
↓ | — | ↓ | State study type |
| For laboratory bioassay. Did the study use standard‐WHO protocol? | ↓ | — | ↓ | |
| For experimental hut study and village trial. Was the study conducted in Africa? | ↓ | — | ↓ | State country |
| Outcome | ||||
Did the study include at least one of the following outcome measures?
|
↓ | — | ↓ | |
| Decision | ||||
| Is the study eligible for inclusion? | — | — | ↓ | State reason(s) for exclusion |
| Discuss with authors | ||||
Abbreviations: ITN: insecticide‐treated net; LLIN: long‐lasting insecticidal net; PBO: piperonyl butoxide; WHO: World Health Organization.
Data extraction and management
After selection, we summarized all included trials according to the tables in Appendix 2. Two review authors (KG and NL) independently extracted data from the included trials using the pre‐designed data extraction form (Appendix 3). If data were missing from an included trial, we contacted the trial authors for further information. We entered data into Review Manager 5 (RevMan 5) (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (KG and NL) independently assessed the risk of bias of each included trial using a set of predetermined criteria specific to each trial type adapted from Strode 2014 (Appendix 4). We assigned a classification of either low, high, or unclear risk of bias for each component. For all included trials we assessed whether any trial authors had submitted any conflicts of interest that may have biased the trial methodology or results.
Laboratory bioassays
For laboratory bioassays we assessed five criteria: comparability of mosquitoes between LLIN/pyrethroid‐PBO net arms (for example, all female, same age, and non‐blood‐fed), observers blinded, incomplete outcome data, raw data reported for both net treatments, and trial authors' conflicting interests.
Experimental hut trials
For experimental hut trials we assessed 11 criteria: comparability of mosquitoes between LLIN/pyrethroid‐PBO net arms (for example, species composition), collectors blinded, sleepers blinded, sleeper bias accounted for, treatment allocation, treatment rotation, standardized hut design, hut cleaning between treatments, incomplete outcome data, raw data reported, and trial authors' conflicting interests.
Village trials
We assessed 12 criteria for village trials: recruitment bias, comparability of mosquitoes between LLIN/pyrethroid‐PBO net households (for example, species composition), collectors blinded, household blinded, treatment allocation, allocation concealment, incomplete outcome data, raw data reported, clusters lost to follow‐up, selective reporting, adjusting for data clustering, and trial authors' conflicting interests.
Measures of treatment effect
For dichotomous data we presented the risk ratio (RR). There were no continuous or count data; however if there had been, we would have used the mean difference and rate ratios, respectively. We have presented all results with 95% confidence intervals (CIs).
Unit of analysis issues
For trials randomized by hut or village, we used the adjusted measure of effect reported in the paper if available. If not reported, we adjusted the effect estimate using an intracluster correlation coefficient (ICC), and average cluster size (Higgins 2011, Section 16.3.4). If the included trial did not report the ICC value, we estimated the ICC value and investigated the impact of estimating the ICC in sensitivity analyses.
To adjust results of experimental hut trials for clustering, we treated each ‘hut and sleeper' combination as the unit of analysis, as each ‘hut and sleeper' combination was tested with each type of net, over a series of nights. We calculated effective sample sizes by estimating an ICC and corresponding design effect. We divided both the number of mosquitoes and the number experiencing the event by this design effect.
To adjust results of village trials for clustering (for which the trial authors had not adjusted the data themselves), we treated each village as the unit of analysis. We calculated effective sample sizes by estimating an ICC and corresponding design effect. Forest plots for both hut and village trials show the effective number of events after adjusting for clustering.
Dealing with missing data
In the case of missing data, we contacted the trial authors to retrieve this information. If we had identified trials where participants were lost to follow‐up, we would have investigated the impact of missing data via imputation using a best‐worst case scenario analysis.
Where information on mosquito insecticide resistance was not collected at the time of the trial, the review authors determined a suitable proxy. The proxy resistance data had to be from the same area, conducted within three years of the trial, and using the same insecticide, dose, and mosquito species. More than 50 mosquitoes per insecticide should have been tested using an appropriate control. Where no resistance data were available, we classed the resistance status as unclassified.
Assessment of heterogeneity
We presented the results of the included trials in forest plots, which we inspected visually to assess heterogeneity (that is, non‐overlapping CIs generally signify statistical heterogeneity). We also used the Chi2 test with a P value of less than 0.1 to indicate statistical heterogeneity. We quantified heterogeneity using the I2 statistic (Higgins 2003), and we interpreted a value greater than 75% to indicate considerable heterogeneity (Deeks 2017).
Assessment of reporting biases
To analyse the possibility of publication bias, we intended to use funnel plots if 10 trials were included in any of the meta‐analyses. However, no analyses included 10 or more trials, so this was not applicable.
Data synthesis
Where appropriate, we pooled the results of the included trials using meta‐analysis. We stratified results by type of trial, mosquito resistance status and net type (i.e. product, for example Olyset Plus).
Three review authors (KG, NL, and MR) analysed the data using RevMan 5 (Review Manager 2014), and we used the random‐effects model (if we detected heterogeneity; the I2 statistic value was more than 75%), or the fixed‐effect model (for no heterogeneity; the I2 statistic value was less than 75%). We would have not pooled trials in meta‐analysis if it was not clinically meaningful to do so, due to clinical or methodological heterogeneity.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to whether nets were washed or unwashed.
Sensitivity analysis
We intended to perform sensitivity analyses to determine the effect of exclusion of trials that we considered to be at high risk of bias, however this was not applicable. We would also have performed a sensitivity analysis for missing data during imputation with best‐worst case scenarios, but again this was not applicable.
We performed sensitivity analyses to investigate the impact of estimating an ICC to adjust trial results for clustering. We performed analyses using ICCs of 0.01, 0.05, and 0.1. Since the results were robust to these adjustments, we used the most conservative ICC (0.1), and we adjusted all results from unadjusted cluster trials using this ICC. We have not presented analyses using the smaller ICCs (0.01 and 0.05).
Certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013). We constructed ‘Summary of findings' tables using the GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT 2015).
Results
Description of studies
Results of the search
We identified 168 records through the electronic searches, and 19 additional records from other sources. We removed duplicates, leaving 175 records, and screened all articles for possible inclusion. After abstract and title screening, we excluded 154 obviously ineligible trials. We assessed 22 full‐text articles for eligibility and excluded seven articles for the following reasons: five trials did not share the full datasets and two studies are ongoing. Fifteen trials met the inclusion criteria (Figure 1).
1.
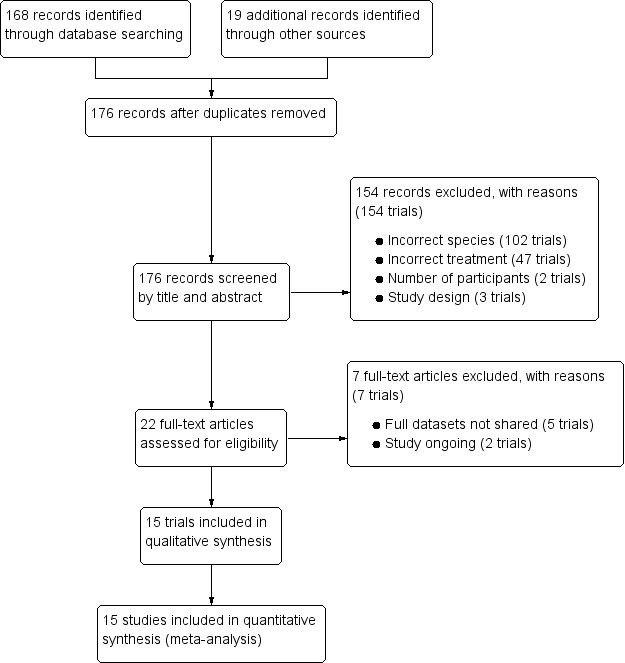
Study flow diagram
Included studies
Fifteen trials met the inclusion criteria, and we have described them in the ‘Characteristics of included studies' tables. Two trials were laboratory bioassays (Darriet 2011; Darriet 2013), eight trials were experimental hut trials (Bayili 2017 (Burkina Faso); Corbel 2010 (Burkina Faso, Benin, Cameroon); Koudou 2011 (Côte d'Ivoire); Moore 2016 (Tanzania); N'Guessan 2010 (Benin); Pennetier 2013 (Benin); Toé 2018 (Burkina Faso); Tungu 2010 (Tanzania)), and five trials were village trials (Awolola 2014 (Nigeria); Cisse 2017 (Mali); Mzilahowa 2014 (Malawi); Protopopoff 2018 (Tanzania); Stiles‐Ocran 2013 (Ghana)). All trials were conducted in Africa.
Interventions
Two trials compared a net treated with deltamethrin to a net treated with deltamethrin and PBO (Darriet 2011; Darriet 2013). Six trials compared Permanet 2.0 to Permanet 3.0 (Awolola 2014; Corbel 2010; Koudou 2011; N'Guessan 2010; Stiles‐Ocran 2013; Tungu 2010). Two trials compared Olyset Net to Olyset Plus (Pennetier 2013; Protopopoff 2018). One trial compared MAGNet LN to Veeralin LN (Moore 2016). Three trials compared both Olyset Net to Olyset Plus and Permanet 2.0 to Permanet 3.0 (Cisse 2017; Mzilahowa 2014; Toé 2018). One trial compared DawaPlus 2.0 to DawaPlus 3.0 and DawaPlus 4.0 (Bayili 2017).
Excluded studies
We assessed 22 full‐text articles for eligibility and excluded seven articles for the following reasons: five trials are awaiting classification because we were unable to obtain the full data sets after we contacted the trial authors (see ‘Characteristics of studies awaiting classification' table), and two trials are ongoing (see ‘Characteristics of ongoing studies' section).
Risk of bias in included studies
We have provided a ‘Risk of bias' assessment summary in Figure 2. The criteria we used to assess risk of bias are in Appendix 5 (laboratory bioassays), Appendix 6 (experimental hut trials), and Appendix 7 (village trials).
2.
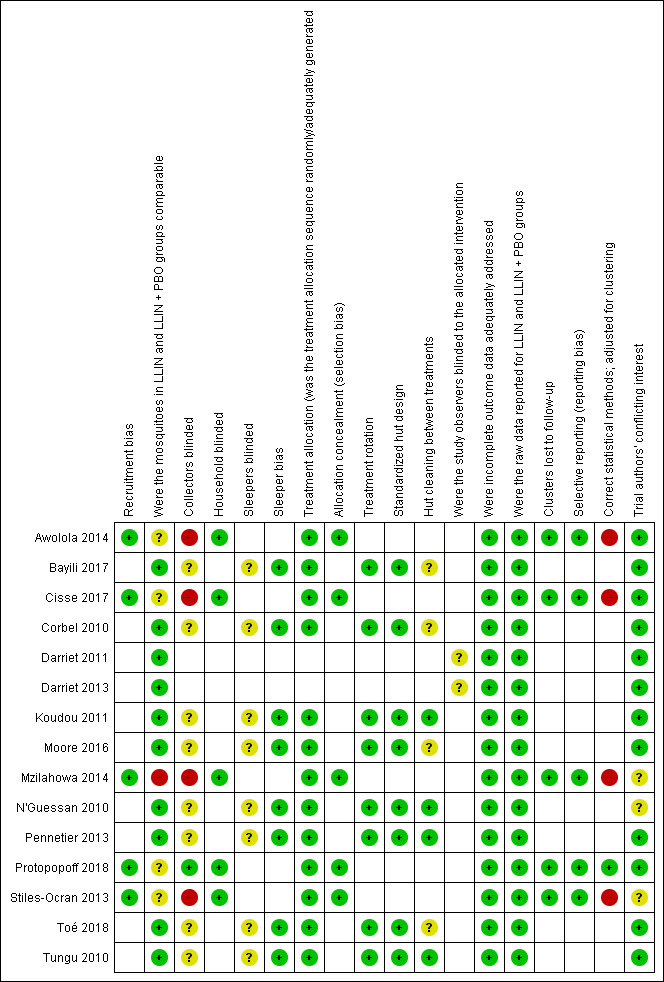
‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Recruitment bias
We assessed all five village trials as at low risk of bias. Four village trials were at low risk of recruitment bias as recruitment bias is related to human participants and so not applicable to this review (Awolola 2014; Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013). We assessed one village trial as low risk as no participants were recruited after clusters had been randomized (Protopopoff 2018).
Mosquito group comparability
We judged the two laboratory bioassays at low risk of bias (Darriet 2011; Darriet 2013), as they used the same mosquito strain, and all eight experimental hut trials at low risk (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), as the huts were situated in the same trial area and therefore accessible to the same mosquito populations. We judged four village trials at unclear risk (Awolola 2014; Cisse 2017; Protopopoff 2018; Stiles‐Ocran 2013), as the species composition and resistance status varied slightly between treatment arms. We deemed one village trial high risk (Mzilahowa 2014), as species and resistance data were not separated by village, and it was not possible to ascertain this from the information provided.
Blinding
We assessed the two laboratory trials (Darriet 2011; Darriet 2013), and eight hut trials (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), as at unclear risk as they did not specify whether observers (lab trials), or collectors and sleepers (hut trials) were blinded. This is not standard protocol for these trial designs and is thought unlikely to affect the results. We judged four village trials at high risk of bias, as it was not stated if collectors were blinded, and this may affect searching effort during collections (Awolola 2014; Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013). We classed one trial as low risk because collectors were masked to the treatment (Protopopoff 2018). For household blinding we judged all five village trials as low risk of bias. Four village trials did not state if households were blind to the intervention, however this was unlikely to influence the results (Awolola 2014; Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013). We judged one village trial as low risk, as inhabitants and field collectors were blinded to intervention arms (Protopopoff 2018).
Sleeper bias
We assessed the eight hut trials at low risk for sleeper bias, as sleepers were rotated between huts following a Latin square design (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010).
Treatment allocation, rotation, and concealment
We assessed the eight hut trials at low risk for treatment allocation and rotation (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), as treatments were rotated between huts following a Latin square design. We assessed five village trials at low risk for treatment allocation (Awolola 2014; Cisse 2017; Mzilahowa 2014; Protopopoff 2018; Stiles‐Ocran 2013), as villages were randomly assigned to treatment arms. We assessed all five village trials as low risk of bias for allocation concealment (Awolola 2014; Cisse 2017; Mzilahowa 2014; Protopopoff 2018; Stiles‐Ocran 2013).
Hut design
We assessed eight hut trials at low risk of bias, as huts were built to standard West‐ or East‐African specifications (Bayili 2017; Corbel 2010; Koudou 2011; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), or using modified but standardized designs (Moore 2016).
Cleaning
We assessed four hut trials at unclear risk as they did not state if huts were cleaned between treatment arms (Bayili 2017; Corbel 2010; Moore 2016; Toé 2018; Tungu 2010). We assessed four at low risk, as cleaning was conducted between treatment rotations (Koudou 2011; N'Guessan 2010; Pennetier 2013; Tungu 2010).
Incomplete outcome data
We assessed all laboratory (Darriet 2011; Darriet 2013), hut (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), and village trials (Awolola 2014; Cisse 2017; Mzilahowa 2014; Protopopoff 2018; Stiles‐Ocran 2013), as at low risk for both incomplete outcome data and raw data reporting, as there were no incomplete outcome data, or missing data were later provided by the trial authors. In cases where raw data were not reported, we were able to calculate them from the percentages and sample sizes given. When these data were not available, we did not include the trials.
Clustering bias
No clusters were lost to follow‐up in any of the village trials, therefore we assessed all village trials as at low risk of bias (Awolola 2014; Cisse 2017; Mzilahowa 2014; Protopopoff 2018; Stiles‐Ocran 2013). We assessed four village trials as at high risk of bias for statistical methods used, as they did not adjust for clustering (Awolola 2014; Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013). We assessed one trial as low risk of bias, as it took clustering into account and adjusted for it in its statistical methods (Protopopoff 2018).
Selective reporting
We assessed all village trials as low risk of bias regarding selective reporting, as they appear to have reported all measured outcomes (Awolola 2014; Cisse 2017; Mzilahowa 2014; Protopopoff 2018; Stiles‐Ocran 2013).
Other potential sources of bias
Conflicting interests
We judged two lab trials (Darriet 2011; Darriet 2013), seven hut trials (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; Pennetier 2013; Toé 2018; Tungu 2010), and three village trials (Awolola 2014; Cisse 2017; Protopopoff 2018), as at low risk as the trial authors reported no conflicting interests. We assessed one hut trial at unclear risk (N'Guessan 2010), as the trial authors stated that they had received funding from LLIN manufacturers when conducting the trials, and the same funders also provided comments on the manuscript. We assessed one village trial as unclear risk, as the trial authors did not state if there were conflicting interests or not (Mzilahowa 2014), and another one as unclear risk, as the trial was conducted to form part of the manufacturer's product dossier (Stiles‐Ocran 2013).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. ‘Summary of findings' table 1.
| Pyrethroid‐piperonyl butoxide (PBO) nets compared to long‐lasting insecticidal nets (LLIN) for malaria control where insecticide resistance is high | ||||||
| Patient or population:Anopheles gambiae complex or Anopheles funestus group Setting: areas of high insecticide resistance Intervention: pyrethroid‐PBO nets Comparison: LLIN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with LLIN | Risk with pyrethroid‐PBO nets | |||||
| Prevalence of malaria | 527 per 1000a | 211 per 1000 (105 to 422)a | OR 0.40 (0.20 to 0.80) | 3966 people (1 trial, 1 comparison) | ⊕⊕⊕⊝
MODERATEb,c due to indirectness |
Prevalence of malaria is probably decreased with pyrethroid‐PBO nets compared to standard LLINs in areas of high insecticide resistance. |
| Mosquito mortality (unwashed nets) | 238 per 1000a | 438 per 1000 (381 to 503)a | RR 1.84 (1.60 to 2.11) | 14,620 mosquitoes (5 trials, 9 comparisons) | ⊕⊕⊕⊕ HIGHb | Mosquito mortality is increased with unwashed pyrethroid‐PBO nets compared to standard unwashed LLINs in areas of high insecticide resistance. |
| Mosquito mortality (washed nets) | 201 per 1000a | 242 per 1000 (177 to 328)a | RR 1.20 (0.88 to 1.63) | 10,268 mosquitoes (4 trials, 5 comparisons) | ⊕⊝⊝⊝
VERY LOWd,e due to imprecision and inconsistency |
We do not know if pyrethroid‐PBO nets have an effect on mosquito mortality in areas of high insecticide resistance when the nets have been washed. |
| Blood‐feeding success (unwashed nets) | 438 per 1000a | 263 per 1000 (241 to 311)a | RR 0.60 (0.50 to 0.71) |
14,000 mosquitoes (4 trials, 8 comparisons) | ⊕⊕⊕⊕ HIGHb | Mosquito blood‐feeding success is decreased with unwashed pyrethroid‐PBO nets compared to standard unwashed LLINs in areas of high insecticide resistance. |
| Blood‐feeding success (washed nets) | 494 per 1000a | 400 per 1000 (356 to 454)a | RR 0.81 (0.72 to 0.92) | 9674 mosquitoes (3 trials, 4 comparisons) | ⊕⊕⊕⊕ HIGHb | Mosquito blood‐feeding success is decreased with washed pyrethroid‐PBO nets compared to standard washed LLINs in areas of high insecticide resistance. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; LLIN: long‐lasting insecticidal nets; OR: odds ratio; PBO: pyrethroid‐piperonyl butoxide; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOriginal numbers used in this table, however in pooled analysis events and total numbers were generated from cluster‐adjusted results which uses the effective sample size. Note that cluster adjustments do not change the point estimate of the effect size, just the standard error. bNot downgraded for imprecision: both best and worst case scenarios in this situation are important effects. cDowngraded by one for indirectness: the outcome is highly context specific and there is only one trial included here. dDowngraded by two for inconsistency due to unexplained qualitative heterogeneity. eDowngraded by one for imprecision due to wide CIs.
Summary of findings 2. ‘Summary of findings' table 2.
| Pyrethroid‐piperonyl butoxide (PBO) nets compared to long‐lasting insecticidal nets (LLIN) for malaria control where insecticide resistance is moderate | ||||||
| Patient or population:Anopheles gambiae complex or Anopheles funestus group Setting: areas of moderate insecticide resistance Intervention: pyrethroid‐PBO nets Comparison: LLIN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of mosquitoes (experimental hut trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with LLIN | Risk with pyrethroid‐PBO nets | |||||
| Mosquito mortality (unwashed nets) | 439 per 1000a | 509 per 1000 (386 to 675)a | RR 1.16 (0.88 to 1.54) | 242 (1 trial, 1 comparison) | ⊕⊕⊝⊝
LOWb,c,d due to imprecision and indirectness |
There may be little or no difference in the effect of unwashed pyrethroid‐PBO nets on mosquito mortality compared to standard unwashed LLINs in areas of moderate insecticide resistance. |
| Mosquito mortality (washed nets) | 287 per 1000a | 307 per 1000 (213 to 443)a | RR 1.07 (0.74 to 1.54) | 329 (1 trial, 1 comparison) | ⊕⊕⊝⊝
LOWb,c,d due to imprecision and indirectness |
There may be little or no difference in the effect of washed pyrethroid‐PBO nets on mosquito mortality compared to standard washed LLINs (washed) in areas of moderate insecticide resistance. |
| Blood‐feeding success (unwashed nets) | 553 per 1000a | 481 per 1000 (370 to 624)a | RR 0.87 (0.67 to 1.13) | 242 (1 trial, 1 comparison) | ⊕⊕⊝⊝
LOWb,c,d due to imprecision and indirectness |
There may be little or no difference in the effect of pyrethroid‐PBO nets (unwashed) on mosquito blood‐feeding success compared to standard LLINs in areas of moderate insecticide resistance. |
| Blood‐feeding success (washed nets) | 586 per 1000a | 533 per 1000 (434 to 662)a | RR 0.91 (0.74 to 1.13) | 329 (1 trial, 1 comparison) | ⊕⊕⊝⊝
LOWb,c,d due to imprecision and indirectness |
There may be little or no difference in the effect of washed pyrethroid‐PBO nets on mosquito blood‐feeding success compared to standard washed LLINs in areas of moderate insecticide resistance. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; LLIN: long‐lasting insecticidal nets; PBO: pyrethroid‐piperonyl butoxide; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOriginal numbers used in this table, however in pooled analysis, we generated events and total numbers from cluster‐adjusted results, which used the effective sample size. Note that cluster adjustments do not change the point estimate of the effect size, just the standard error. bNot downgraded for inconsistency as only one trial measured this outcome in this setting. cDowngraded by one for imprecision due to wide CIs. dDowngraded by one for indirectness: the outcome is highly context‐specific and there is only one trial included here.
Summary of findings 3. ‘Summary of findings' table 3.
| Pyrethroid‐piperonyl butoxide (PBO) nets compared to long‐lasting insecticidal nets (LLIN) for malaria control where insecticide resistance is low | ||||||
| Patient or population:Anopheles gambiae complex or Anopheles funestus group Setting: areas of low insecticide resistance Intervention: pyrethroid‐PBO nets Comparison: LLIN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of mosquitoes (experimental hut trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with LLIN | Risk with pyrethroid‐PBO nets | |||||
| Mosquito mortality (unwashed nets) | 871 per 1000a | 958 per 1000 (914 to 1000)a | RR 1.10 (1.05 to 1.16) | 708 (1 trial, 2 comparisons) | ⊕⊕⊕⊝
MODERATEb due to imprecision |
There is probably little or no difference in the effect of unwashed pyrethroid‐PBO nets on mosquito mortality compared to standard unwashed LLINs in areas of low insecticide resistance. |
| Mosquito mortality (washed nets) | 620 per 1000a | 719 per 1000 (514 to 1000)a | RR 1.16 (0.83 to 1.63) | 878 (1 trial, 2 comparisons) | ⊕⊝⊝⊝
VERY LOWc,d due to imprecision and inconsistency |
We do not know if pyrethroid‐PBO nets have an effect on mosquito mortality in areas of low insecticide resistance when the nets have been washed. |
| Blood‐feeding success (unwashed nets) | 72 per 1000a | 48 per 1000 (4 to 529)a | RR 0.67 (0.06 to 7.37) | 708 (1 trial, 2 comparisons) | ⊕⊝⊝⊝
VERY LOWc,d due to imprecision and inconsistency |
We do not know if unwashed pyrethroid‐PBO nets have an effect on mosquito blood‐feeding success in areas of low insecticide resistance. |
| Blood‐feeding success (washed nets) | 149 per 1000a | 223 per 1000 (132 to 377)a | RR 1.50 (0.89 to 2.54) | 878 (1 trial, 2 comparisons) | ⊕⊕⊝⊝
LOWd due to inconsistency |
Mosquito blood‐feeding success may decrease with washed pyrethroid‐PBO nets compared to standard washed LLINs in areas of low insecticide resistance. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; LLIN: long‐lasting insecticidal nets; PBO: pyrethroid‐piperonyl butoxide; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOriginal numbers used in this table, however in pooled analysis events and total numbers were generated from cluster adjusted results which uses the effective sample size. Note that cluster adjustments do not change the point estimate of the effect size, just the standard error. bDowngraded by one for imprecision due to wide CIs. cDowngraded by one for inconsistency due to unexplained heterogeneity. dDowngraded by two for imprecision due to extremely wide CIs.
Summary of findings 4. ‘Summary of findings' table 4.
| Pyrethroid‐piperonyl butoxide (PBO) nets compared to long‐lasting insecticidal nets (LLIN) for malaria control where mosquitoes are susceptible | ||||||
|
Patient or population:Anopheles gambiae complex or Anopheles funestus group
Setting: areas of insecticide‐susceptible mosquitoes Intervention: pyrethroid‐PBO nets Comparison: LLIN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of mosquitoes (experimental hut trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with LLIN | Risk with pyrethroid‐PBO nets | |||||
| Mosquito mortality (unwashed nets) | 392 per 1000a | 471 per 1000 (251 to 887)a | RR 1.20 (0.64 to 2.26) | 2791 (2 trials, 2 comparisons) | ⊕⊕⊝⊝
LOWb due to imprecision |
There may be little or no difference in the effect of unwashed pyrethroid‐PBO nets on mosquito mortality compared to standard unwashed LLINs in areas of no insecticide resistance. |
| Mosquito mortality (washed nets) | 457 per 1000a | 489 per 1000 (420 to 571)a | RR 1.07 (0.92 to 1.25) | 2644 (2 trials, 2 comparisons) | ⊕⊕⊝⊝
LOWb due to imprecision |
There may be little or no difference in the effect of washed pyrethroid‐PBO nets on mosquito mortality compared to standard washed LLINs in areas of no insecticide resistance. |
| Blood‐feeding success (unwashed nets) | 57 per 1000a | 29 per 1000 (6 to 132)a | RR 0.50 (0.11 to 2.32) | 2791 (2 trials, 2 comparisons) | ⊕⊝⊝⊝
VERY LOWb,c due to imprecision and inconsistency |
We do not know if unwashed pyrethroid‐PBO nets have an effect on mosquito blood‐feeding success in areas of no insecticide resistance. |
| Blood‐feeding success (washed nets) | 64 per 1000a | 82 per 1000 (52 to 131)a | RR 1.28 (0.81 to 2.04) | 2644 (2 trials, 2 comparisons) | ⊕⊕⊝⊝
LOWb due to imprecision |
There may be little or no difference in the effect of washed pyrethroid‐PBO nets on mosquito blood‐feeding success compared to standard washed LLINs in areas of no insecticide resistance. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; LLIN: long‐lasting insecticidal nets; PBO: pyrethroid‐piperonyl butoxide; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOriginal numbers used in this table, however in pooled analysis, events and total numbers were generated from cluster‐adjusted results, which use the effective sample size. Note that cluster adjustments do not change the point estimate of the effect size, just the standard error. bDowngraded by two for imprecision due to extremely wide CIs. cDowngraded by one for inconsistency due to unexplained heterogeneity.
Objective 1
Objective 1 was to evaluate whether adding PBO to pyrethroid‐LLINs increases the epidemiological and entomological effectiveness of bed nets.
Epidemiological results
None of the trials that met the inclusion criteria for Objective 1 measured parasite presence, or clinical malaria confirmation, in adults or children.
Entomological results
Two laboratory trials evaluated the impact of an insecticide‐treated net (ITN), impregnated with deltamethrin (a pyrethroid insecticide), only, compared to ITN plus PBO (PBO‐ITN), on mosquito mortality in an insecticide‐resistant laboratory strain (Darriet 2011; Darriet 2013). The pooled analysis showed substantially increased mosquito mortality when using pyrethroid‐PBO (RR 6.06, 95% CI 4.15 to 8.84; 558 mosquitoes, 2 trials; Analysis 1.1).
1.1. Analysis.
Comparison 1 Pyrethroid‐PBO nets versus LLINs: laboratory bioassay (cone trials), Outcome 1 Mosquito mortality.
Objective 2
Objective 2 compared the effects of pyrethroid‐PBO nets currently in commercial development or on the market with their non‐PBO equivalent in relation to malaria infection and entomological outcomes.
Epidemiological results
One trial examined the effect of pyrethroid‐PBO nets on malaria infection prevalence (Protopopoff 2018). Overall the latest endpoint at 21 months after the intervention showed that malaria prevalence decreased in the intervention arm (OR 0.40, 95% CI 0.20 to 0.80; 1 trial, 1 comparison; Analysis 3.1).
3.1. Analysis.
Comparison 3 Commercial pyrethroid‐PBO nets versus commercial LLINs: village trials, Outcome 1 Prevalence of malaria.
Entomological results
Phase II experimental hut trials
Eight experimental hut trials (phase II trials), examined the effect of pyrethroid‐PBO nets on mosquito mortality, blood feeding, exophily, and deterrence (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010). We subgrouped the data by net washing, into unwashed and washed groups. Washed nets were all washed 20 times according to WHO specifications (WHO 2013). We pooled the results initially and then stratified them by insecticide resistance level and net type. One trial did not wash their nets and so did not report any data for the washed subgroup (Toé 2018). One trial did not introduce holes into the nets and so did not report blood‐feeding success data (Koudou 2011).
Pooled analysis
Pooled analysis of all experimental hut trials, using both unwashed (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010) and washed nets (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Tungu 2010), found that pyrethroid‐PBO nets significantly increased mosquito mortality, by 36% (RR 1.36, 95% CI 1.20 to 1.54), and reduced blood‐feeding success by 24% (RR 0.76, 95% CI 0.67 to 0.88). The magnitude of effect was reduced by net washing. Unwashed pyrethroid‐PBO nets increased mosquito mortality by 54% compared to unwashed LLINs, (RR 1.54, 95% CI 1.21 to 1.96; 8 trials, 15 comparisons; Analysis 2.1); when nets are washed this effect decreased to 14%, (RR 1.14, 95% 1.00 to 1.31; 7 trials, 11 comparisons; Analysis 2.1). Unwashed pyrethroid‐PBO nets reduced mosquito blood‐feeding success by 34% (Bayili 2017; Corbel 2010; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010; RR 0.66, 95% CI 0.54 to 0.79; 7 trials, 14 comparisons; Analysis 2.2); however this effect was lost when nets were washed (Bayili 2017; Corbel 2010; Moore 2016; N'Guessan 2010; Pennetier 2013; Tungu 2010; 6 trials, 10 comparisons; Analysis 2.2). There was no effect on mosquito exophily in either unwashed (8 trials, 15 comparisons; Analysis 2.3) or washed (7 trials, 11 comparisons; Analysis 2.3), groups. Mosquito deterrence data were presented relative to an untreated control and hence are not included as a forest plot. There was considerable variation in the deterrence rates but no clear relationship with resistance level, net type, or washing status (Table 11).
2.1. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 1 Mosquito mortality (pooled) hut/night.
2.2. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 2 Mosquito blood‐feeding success (pooled) hut/night.
2.3. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 3 Mosquito exophily (pooled) hut/night.
7. Experimental hut trials: deterrence data.
| Study ID | Locality | Net type | Net washed | Total number in ITN hut | Total number in UTN hut | Deterrence (%) reported | Deterrence (%) calculated |
| Bayili 2017 | Vallée du Kou | DawaPlus 2.0 | No | 1548 | 1848 | 16.23 | 16.23 |
| Bayili 2017 | Vallée du Kou | DawaPlus 2.0 | Yes | 2155 | 1848 | 0 | ‐16.61 |
| Bayili 2017 | Vallée du Kou | DawaPlus 3.0 | No | 1365 | 1848 | 26.13 | 26.14 |
| Bayili 2017 | Vallée du Kou | DawaPlus 3.0 | Yes | 1981 | 1848 | 0 | ‐7.20 |
| Bayili 2017 | Vallée du Kou | DawaPlus 4.0 | No | 846 | 1848 | 54.22 | 54.22 |
| Bayili 2017 | Vallée du Kou | DawaPlus 4.0 | Yes | 1646 | 1848 | 10.93 | 10.93 |
| Corbel 2010 | Malanville | Permanet 2.0 | Yes | 195 | 285 | 31.58 | 31.58 |
| Corbel 2010 | Malanville | Permanet 3.0 | Yes | 210 | 285 | 26.32 | 26.32 |
| Corbel 2010 | Malanville | Permanet 2.0 | No | 243 | 285 | 14.74 | 14.74 |
| Corbel 2010 | Malanville | Permanet 3.0 | No | 214 | 285 | 24.91 | 24.91 |
| Corbel 2010 | Pitoa | Permanet 2.0 | Yes | 310 | 401 | 22.69 | 22.69 |
| Corbel 2010 | Pitoa | Permanet 3.0 | Yes | 163 | 401 | 59.35 | 59.35 |
| Corbel 2010 | Pitoa | Permanet 2.0 | No | 105 | 401 | 73.82 | 73.82 |
| Corbel 2010 | Pitoa | Permanet 3.0 | No | 146 | 401 | 63.59 | 63.59 |
| Corbel 2010 | Vallée du Kou | Permanet 2.0 | Yes | 788 | 908 | 13.22 | 13.22 |
| Corbel 2010 | Vallée du Kou | Permanet 3.0 | Yes | 724 | 908 | 20.26 | 20.26 |
| Corbel 2010 | Vallée du Kou | Permanet 2.0 | No | 329 | 908 | 63.77 | 63.77 |
| Corbel 2010 | Vallée du Kou | Permanet 3.0 | No | 463 | 908 | 49.01 | 49.01 |
| Koudou 2011 | Yaokoffikro | Permanet 3.0 | No | 303 | 796 | 62.1 | 61.93 |
| Koudou 2011 | Yaokoffikro | Permanet 2.0 | No | 317 | 796 | 60.4 | 60.18 |
| Koudou 2011 | Yaokoffikro | Permanet 3.0 | Yes | 313 | 796 | 60.1 | 60.68 |
| Koudou 2011 | Yaokoffikro | Permanet 2.0 | Yes | 281 | 796 | 64.4 | 64.70 |
| Moore 2016 | Ifakara | Veeralin LN | No | 722 | 810 | 11 | 10.86 |
| Moore 2016 | Ifakara | Veeralin LN | Yes | 727 | 810 | 10 | 10.25 |
| Moore 2016 | Ifakara | MAGNet LN | No | 1070 | 810 | 0 | ‐32.10 |
| Moore 2016 | Ifakara | MAGNet LN | Yes | 773 | 810 | 5 | 4.57 |
| Moore 2016 | Ifakara | Veeralin LN | No | 89 | 170 | 48 | 47.65 |
| Moore 2016 | Ifakara | Veeralin LN | Yes | 85 | 170 | 50 | 50.00 |
| Moore 2016 | Ifakara | MAGNet LN | No | 114 | 170 | 33 | 32.94 |
| Moore 2016 | Ifakara | MAGNet LN | Yes | 103 | 170 | 39 | 39.41 |
| N'Guessan 2010 | Akron | Permanet 3.0 | No | 128 | 185 | 31 | 30.81 |
| N'Guessan 2010 | Akron | Permanet 3.0 | Yes | 155 | 185 | NR | 16.22 |
| N'Guessan 2010 | Akron | Permanet 2.0 | No | 114 | 185 | 38 | 38.38 |
| N'Guessan 2010 | Akron | Permanet 2.0 | Yes | 174 | 185 | NR | 5.95 |
| Pennetier 2013 | Malanville | Olyset Plus | No | 67 | 69 | NR | 2.90 |
| Pennetier 2013 | Malanville | Olyset Plus | Yes | 101 | 69 | NR | ‐46.38 |
| Pennetier 2013 | Malanville | Olyset Net | No | 96 | 69 | NR | ‐39.13 |
| Pennetier 2013 | Malanville | Olyset Net | Yes | 124 | 69 | NR | ‐79.71 |
| Toé 2018 | Tengrela | Olyset Net | No | 923 | 480 | ‐92.29 | ‐92.29 |
| Toé 2018 | Tengrela | Olyset Plus | No | 695 | 480 | ‐44.79 | ‐44.79 |
| Toé 2018 | Tengrela | Permanet 2.0 | No | 858 | 480 | ‐78.75 | ‐78.75 |
| Toé 2018 | Tengrela | Permanet 3.0 | No | 794 | 480 | ‐65.42 | ‐65.42 |
| Toé 2018 | VK5 | Olyset Net | No | 1458 | 1095 | ‐33.15 | ‐33.15 |
| Toé 2018 | VK5 | Olyset Plus | No | 1278 | 1095 | ‐16.71 | ‐16.71 |
| Toé 2018 | VK5 | Permanet 2.0 | No | 1075 | 1095 | 1.83 | 1.83 |
| Toé 2018 | VK5 | Permanet 3.0 | No | 657 | 1095 | 40 | 40.00 |
| Tungu 2010 | Zeneti | PermaNet 3.0 | No | 425 | 723 | 41 | 41.22 |
| Tungu 2010 | Zeneti | PermaNet 2.0 | No | 574 | 723 | 21 | 20.61 |
| Tungu 2010 | Zeneti | PermaNet 3.0 | Yes | 558 | 723 | 23 | 22.82 |
| Tungu 2010 | Zeneti | PermaNet 2.0 | Yes | 586 | 723 | 19 | 18.95 |
Abbreviations: ITN: insecticide‐treated net; LLIN: long‐lasting insecticidal net; NR: not reported; PBO: piperonyl butoxide; UTN: untreated net; WHO: World Health Organization.
There was considerable heterogeneity in this pooled analysis, particularly for estimates of mortality. We therefore performed a pre‐specified, stratified analysis, dividing the results into trials conducted in areas of low, moderate, or high resistance in the Anopheles population.
Stratified analysis: mosquito resistance status
We used the WHO and CDC definitions of mosquito mortality, from WHO tube assays or CDC bottle tests (Table 8) to classify mosquito resistance. Both tests define mosquitoes as resistant when mortality is less than 90%. We further stratified resistance based on the following mortality levels: < 30%, high resistance; 31% to 60%, moderate resistance; 61% to 90%, low resistance (Table 9). When resistance data were not collected at the time of the trial, we identified a suitable proxy based on previously described criteria (see ‘Dealing with missing data' section); when we could not identify a suitable proxy, we classed the trial as ‘unclassified' and did not include it in the resistance stratification.
Five trials were conducted in four areas where mosquito populations exhibited high resistance to pyrethroids (Bayili 2017; Corbel 2010; Koudou 2011; Pennetier 2013; Toé 2018). Under these conditions unwashed pyrethroid‐PBO nets increased mosquito mortality by 84% in comparison to unwashed LLINs (RR 1.84, 95% CI 1.60 to 2.11; 5 trials, 9 comparisons; Analysis 2.4), however this effect was lost when nets were washed (Bayili 2017; Corbel 2010; Koudou 2011; Pennetier 2013; 4 trials, 5 comparisons; Analysis 2.4). Blood‐feeding success was reduced by 40% in unwashed pyrethroid‐PBO‐net groups compared to unwashed LLINs (Bayili 2017; Corbel 2010; Pennetier 2013; Toé 2018; RR 0.60, 95% CI 0.50 to 0.71; 4 trials, 8 comparisons; Analysis 2.5), and by 19% when nets were washed (Bayili 2017; Corbel 2010; Pennetier 2013; RR 0.81, 95% CI 0.72 to 0.92; 3 trials, 4 comparisons; Analysis 2.5).
2.4. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 4 Mosquito mortality (high resistance) hut/night.
2.5. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 5 Mosquito blood‐feeding success (high resistance) hut/night.
One trial in one site was conducted in an area with moderate insecticide resistance (N'Guessan 2010). No effect on mosquito mortality (1 trial, 1 comparison; Analysis 2.6) or on blood‐feeding success (1 trial, 1 comparison; Analysis 2.7) was observed in either washed or unwashed treatments.
2.6. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 6 Mosquito mortality (moderate resistance) hut/night.
2.7. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 7 Mosquito blood‐feeding success (moderate resistance) hut/night.
One trial in two sites was conducted in an area with low insecticide resistance (Corbel 2010). A small effect on mosquito mortality was observed in unwashed nets (RR 1.10, 95% CI 1.05 to 1.16; 1 trial, 2 comparisons; Analysis 2.8), however this was lost by washing (1 trial, 2 observations; Analysis 2.8). No effect was seen in blood‐feeding success (1 trial, 2 comparisons; Analysis 2.9).
2.8. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 8 Mosquito mortality (low resistance) hut/night.
2.9. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 9 Mosquito blood‐feeding success (low resistance) hut/night.
In susceptible sites (Moore 2016; Tungu 2010), no effect on mosquito mortality (2 trials, 2 comparisons; Analysis 2.10), or blood‐feeding success (2 trials, 2 comparisons; Analysis 2.11), was observed.
2.10. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 10 Mosquito mortality (susceptible) hut/night.
2.11. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 11 Mosquito blood‐feeding success (susceptible) hut/night.
Stratified analysis: net type
After stratifying by resistance status, we performed a secondary analysis stratified according to net type. Due to the limited number of trials, we only performed this analysis for trials using PermaNet 3.0 or Olyset Plus. Although additional trials utilising Veeralin LN, DawaPlus 3.0, and DawaPlus 4.0 have been conducted, not all data were made available to us for the purposes of this Cochrane Review. Futhermore, the analysis was restricted to trials conducted in areas of high resistance, as this analysis only indicated an impact of pyrethroid‐PBO nets in these settings. Three trials compared PermaNet 2.0 (LLIN) to PermaNet 3.0 (pyrethroid‐PBO nets), and two compared Olyset Nets (LLIN) to Olyset Plus (pyrethroid‐PBO nets).
In the PermaNet group, in high‐resistance settings, unwashed PermaNet 3.0 increased mosquito mortality by 81% compared to PermaNet 2.0 (Corbel 2010; Koudou 2011; Toé 2018; RR 1.81, 95% CI 1.56 to 2.10; 3 trials, 4 comparisons; Analysis 2.12). After washing there was no significant increase in mortality in the PermaNet 3.0 arm (Corbel 2010; Koudou 2011; 2 trials, 2 comparisons; Analysis 2.12). Blood‐feeding success was reduced by 47% when using unwashed PermaNet 3.0 (Corbel 2010; Toé 2018; RR 0.53, 95% CI 0.40 to 0.69; 2 trials, 3 comparisons; Analysis 2.13), only one trial was available for washed nets (Corbel 2010), and here PermaNet 3.0 also reduced blood‐feeding success (RR 0.76, 95% 0.61 to 0.93; 1 trial, 1 comparison; Analysis 2.13).
2.12. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 12 Mosquito mortality (high resistance/Permanet) hut/night.
2.13. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 13 Mosquito blood‐feeding success (high resistance/Permanet) hut/night.
In high‐resistance settings, Olyset Plus increased mosquito mortality by 72% when nets were unwashed (Pennetier 2013; Toé 2018; RR 1.72, 95% CI 1.48 to 1.99; 2 trials, 3 comparisons; Analysis 2.14). Only one trial compared washed Olyset Plus with washed Olyset (Pennetier 2013); in this trial the enhanced mortality (81%) in the Olyset Plus arm was still observed after washing (RR 1.81, 95% CI 1.25 to 2.61; 1 trial, 1 comparison; Analysis 2.14). There was no impact on blood‐feeding success when comparing unwashed Olyset Plus with Olyset (2 trials, 3 comparisons; Analysis 2.15); the single trial that looked at washed Olyset Plus showed decreased blood feeding compared to Olyset (RR 0.50, 95% 0.27 to 0.93; 1 trial, 1 comparison; Analysis 2.15)
2.14. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 14 Mosquito mortality (high resistance/Olyset) hut/night.
2.15. Analysis.
Comparison 2 Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials, Outcome 15 Mosquito blood‐feeding success (high resistance/Olyset) hut/night.
Phase III village trials
In the village trials there was no decrease in sporozoite rate in the trial arms receiving pyrethroid‐PBO nets (Awolola 2014; Cisse 2017; Protopopoff 2018; Stiles‐Ocran 2013; RR 0.82, 95% CI 0.24 to 2.75; 4 trials, 5 comparisons; Analysis 3.2). Mosquito parity was not reduced in pyrethroid‐PBO villages (Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013; 3 trials, 4 comparisons; Analysis 3.3). It was not possible to stratify these data by resistance status due to the variability in resistance levels between villages within the same trial. Mosquito density was measured using a variety of methods and summarized in different ways (for example, mean number caught per house, mean number caught per village). Where baseline data were collected, we calculated a percentage reduction. Higher reductions in mosquito densities were observed in pyrethroid‐PBO net villages compared to LLIN villages (Table 12).
3.2. Analysis.
Comparison 3 Commercial pyrethroid‐PBO nets versus commercial LLINs: village trials, Outcome 2 Mosquito sporozoite‐positive (adjusted ICC 0.1).
3.3. Analysis.
Comparison 3 Commercial pyrethroid‐PBO nets versus commercial LLINs: village trials, Outcome 3 Mosquito parous (adjusted ICC 0.1).
8. Village trials: mosquito density data.
| Study ID | Net type | Species | Density measurement | Collection method | Baseline density | Post‐intervention density | Reduction (%) |
| Awolola 2014 | Untreated | An gambiae s.l. | Mean number caught per house | WT, IRC | 16.2 | 17.1 | ‐5.56 |
| Awolola 2014 | PermaNet 2.0 | An gambiae s.l. | Mean number caught per house | WT, IRC | 21.3 | 7.2 | 66.20 |
| Awolola 2014 | PermaNet 3.0 | An gambiae s.l. | Mean number caught per house | WT, IRC | 20.1 | 1.4 | 93.03 |
| Cisse 2017 | PermaNet 2.0 | An gambiae s.l. | Resting density per room per day | IRC | ‐ | 1.92 | ‐ |
| Cisse 2017 | PermaNet 3.0 | An gambiae s.l. | Resting density per room per day | IRC | ‐ | 3.05 | ‐ |
| Cisse 2017 | Olyset | An gambiae s.l. | Resting density per room per day | IRC | ‐ | 3.21 | ‐ |
| Cisse 2017 | Olyset Plus | An gambiae s.l. | Resting density per room per day | IRC | ‐ | 3.7 | ‐ |
| Mzilahowa 2014 | Olyset | An gambiae | Mean number caught per catch | PSC | ‐ | 0.10 | ‐ |
| Mzilahowa 2014 | Olset Plus | An gambiae | Mean number caught per catch | PSC | ‐ | 0.12 | ‐ |
| Mzilahowa 2014 | PermaNet 2.0 | An gambiae | Mean number caught per catch | PSC | ‐ | 0.13 | ‐ |
| Mzilahowa 2014 | PermaNet 3.0 | An gambiae | Mean number caught per catch | PSC | ‐ | 0.09 | ‐ |
| Mzilahowa 2014 | Olyset | An funestus | Mean number caught per catch | PSC | ‐ | 0.08 | ‐ |
| Mzilahowa 2014 | Olyset Plus | An funestus | Mean number caught per catch | PSC | ‐ | 0.16 | ‐ |
| Mzilahowa 2014 | PermaNet 2.0 | An funestus | Mean number caught per catch | PSC | ‐ | 0.27 | ‐ |
| Mzilahowa 2014 | PermaNet 3.0 | An funestus | Mean number caught per catch | PSC | ‐ | 0.13 | ‐ |
| Mzilahowa 2014 | Olyset | An gambiae | Mean number caught per catch | LT | ‐ | 1.23 | ‐ |
| Mzilahowa 2014 | Olset Plus | An gambiae | Mean number caught per catch | LT | ‐ | 0.27 | ‐ |
| Mzilahowa 2014 | PermaNet 2.0 | An gambiae | Mean number caught per catch | LT | ‐ | 0.96 | ‐ |
| Mzilahowa 2014 | PermaNet 3.0 | An gambiae | Mean number caught per catch | LT | ‐ | 1.44 | ‐ |
| Mzilahowa 2014 | Olyset | An funestus | Mean number caught per catch | LT | ‐ | 2.02 | ‐ |
| Mzilahowa 2014 | Olset Plus | An funestus | Mean number caught per catch | LT | ‐ | 2.1 | ‐ |
| Mzilahowa 2014 | PermaNet 2.0 | An funestus | Mean number caught per catch | LT | ‐ | 5.76 | ‐ |
| Mzilahowa 2014 | PermaNet 3.0 | An funestus | Mean number caught per catch | LT | ‐ | 3.76 | ‐ |
| Protopopoff 2018 | Olyset (2015) | Anopheles species | Mean number caught per house per night | LT | ‐ | 2.61 | ‐ |
| Protopopoff 2018 | Olyset Plus (2015) | Anopheles species | Mean number caught per house per night | LT | ‐ | 1.85 | ‐ |
| Protopopoff 2018 | Olyset (2016) | Anopheles species | Mean number caught per house per night | LT | ‐ | 3.60 | ‐ |
| Protopopoff 2018 | Olyset Plus (2016) | Anopheles species | Mean number caught per house per night | LT | ‐ | 2.68 | ‐ |
| Stiles‐Ocran 2013 | No intervention | An gambiae s.s. | Mean number caught per village | IRC | 230 | 79 | 65.65 |
| Stiles‐Ocran 2013 | Permanet 2.0 | An gambiae s.s. | Mean number caught per village | IRC | 39 | 36 | 7.69 |
| Stiles‐Ocran 2013 | Permanet 2.0 | An gambiae s.s. | Mean number caught per village | IRC | 82 | 45 | 45.12 |
| Stiles‐Ocran 2013 | Permanet 3.0 | An gambiae s.s. | Mean number caught per village | IRC | 77 | 12 | 84.42 |
| Stiles‐Ocran 2013 | Permanet 3.0 | An gambiae s.s. | Mean number caught per village | IRC | 178 | 15 | 91.57 |
| Stiles‐Ocran 2013 | No intervention | An gambiae s.s. | Mean number caught per person per night per village | Indoor & outdoor HLC | 415 | 72 | 82.65 |
| Stiles‐Ocran 2013 | Permanet 2.0 | An gambiae s.s. | Mean number caught per person per night per village | Indoor & outdoor HLC | 33 | 31 | 6.06 |
| Stiles‐Ocran 2013 | Permanet 2.0 | An gambiae s.s. | Mean number caught per person per night per village | Indoor & outdoor HLC | 79 | 64 | 18.99 |
| Stiles‐Ocran 2013 | Permanet 3.0 | An gambiae s.s. | Mean number caught per person per night per village | Indoor & outdoor HLC | 98 | 19 | 80.61 |
| Stiles‐Ocran 2013 | Permanet 3.0 | An gambiae s.s. | Mean number caught per person per night per village | Indoor & outdoor HLC | 156 | 36 | 76.92 |
Abbreviations: An gambiae: Anopheles gambiae; An funestus: Anopheles funestus; HLC: human landing catch; IRC: indoor resting catch; LT: light trap; PSC: pyrethrum spray catch; WT: window trap.
Discussion
See Table 1, Table 2, Table 3, and Table 4.
Summary of main results
There was a single cluster‐RCT performed on pyrethroid‐PBO nets. This trial, which compared malaria prevalence in children using Olyset Plus nets with those using Olyset nets, in a region of Tanzania where the mosquito vectors are highly resistant to pyrethroids, found that pyrethroid‐PBO nets reduced malaria prevalence by 60% (Protopopoff 2018). All other trials included in this review measured entomological endpoints. Four villages measured sporozoite rates in mosquitoes collected from houses using pyrethroid‐PBO nets and standard pyrethroid LLINs but the results were highly heterogeneous and there was no evidence that pyrethroid‐PBO nets reduced the mosquito infection rate from this pooled analysis (Awolola 2014; Cisse 2017; Protopopoff 2018; Stiles‐Ocran 2013). Similarly, the proportion of parous mosquitoes (that is, mosquitoes that have survived past one gonotrophic cycle, used as an indirect measure of longevity), was not significantly affected by the presence of pyrethroid‐PBO nets (Cisse 2017; Mzilahowa 2014; Stiles‐Ocran 2013).
When we pooled the results from the eight experimental hut trials (Bayili 2017; Corbel 2010; Koudou 2011; Moore 2016; N'Guessan 2010; Pennetier 2013; Toé 2018; Tungu 2010), the results showed an improved performance of pyrethroid‐PBO LLINs over standard LLINs in both increasing mosquito mortality and reducing blood feeding but the results were highly heterogeneous. Stratifying the experimental hut data by resistance levels in the population reduced the heterogeneity. In areas where mosquitoes are highly resistant to pyrethroids, pyrethroid‐PBO nets will reduce mosquito blood‐feeding rates (that is, users of the nets will be better protected against mosquito bites by using pyrethroid‐PBO nets). This impact on blood feeding is reduced when nets have been through the standard 20 washes recommended by the WHO to assess chemical durability, but remains significant (high‐certainty evidence). When resistance is high and new unwashed nets are used, mosquito mortality is substantially increased when the nets contain PBO compared to pyrethroid‐only LLINs (high‐certainty evidence). However this effect on mosquito mortality, which is important for the community‐level protection afforded by LLIN usage (Hawley 2003; Maxwell 2002), is not sustained when nets have been washed multiple times. In this Cochrane Review we classified mosquitoes as being highly resistant if fewer than 30% were killed in a standard bioassay. When mortality rates exceeded 30%, there was little evidence that pyrethroid‐PBO nets provided greater personal protection or resulted in greater mosquito mortality than standard pyrethroid‐only nets. This result is not unexpected given that, in areas where resistance is uncommon or absent, exposure to pyrethroids alone would be expected to negatively affect the mosquito; it is only in areas where the efficacy of pyrethroids has been eroded by the development of high levels of resistance where the addition of a synergist might be needed.
There was no evidence for any difference in the performance of pyrethroid‐PBO nets from different manufacturers against highly pyrethroid‐resistant mosquitoes. We only stratified results by net type for trials that were conducted in areas of high resistance. We have not reported comparisons for DawaPlus‐PBO and Veeralin‐PBO nets in this subanalysis as there was only a single data point for these net types. Unwashed PermaNet 3.0 and Olyset Plus resulted in similar increases in mosquito mortality compared to pyrethroid‐only LLINs from the same manufacturer although this effect on mortality was not always sustained after washing (Corbel 2010; Koudou 2011; Pennetier 2013; Toé 2018). A significant improvement in personal protection for unwashed pyrethroid‐PBO nets was only observed for PermaNet 3.0 (Corbel 2010; Toé 2018), but, after washing, pyrethroid‐PBO nets from both manufacturers provided greater personal protection than the equivalent pyrethroid‐only nets (Corbel 2010; Pennetier 2013). Results from comparisons between pyrethroid‐PBO nets from different manufacturers should be taken with great caution given the very limited number of data points available, particularly for washed nets. Further trials, where nets from different manufacturers are directly compared in the same trial are needed to address the issue of equivalence between different pyrethroid‐PBO nets.
Certainty of the evidence
We appraised the certainty of the evidence using the GRADE approach (Table 1, Table 2, Table 3, and Table 4). The single RCT provides moderate‐certainty evidence that pyrethroid‐PBO nets reduce the prevalence of malaria. However, this result was obtained from a single setting (Protopopoff 2018). The WHO recommends at least two RCTs in areas with differing levels of malaria transmission to demonstrate public health value (WHO‐GMP 2017b). Hence this Cochrane Review will need to be updated once data from the large‐scale RCT of pyrethroid‐PBO nets in Uganda are available (ISRCTN17516395).
The certainty of evidence from trials using entomological endpoints varied. Data from village trials were difficult to assess as there was considerable heterogeneity in the level of pyrethroid resistance, and presumably also in the resistance mechanisms, both within and between trials. Analysis of data from experimental hut trials found high‐certainty evidence for the superior performance of pyrethroid‐PBO nets in areas of high resistance but the evidence for trials conducted in other settings was of low or very low certainty.
Overall completeness and applicability of evidence
Only two published laboratory trials directly measured the impact of adding PBO to LLINs (Darriet 2011; Darriet 2013). All other trials included in this review compared pyrethroid‐PBO nets with the nearest equivalent pyrethroid‐only LLIN. Further changes to the net specification were often included when the manufacturers incorporated the synergist. For example, the pyrethroid‐PBO net manufactured by Vestergaard (PermaNet 3.0), contains higher levels of deltamethrin and a different denier (thickness) of yarn compared to the pyrethroid‐only equivalent, PermaNet 2.0; the pyrethroid in Olyset Plus (Sumitomo Chemical Co Ltd), is released from the yarn at a different rate than in the Olyset nets. These additional variations in the chemical or physical composition, or both, of the nets makes it difficult to directly assess the added value of the addition of PBO. Furthermore the concentration of PBO and its site of application differs markedly between nets from the different manufacturers. Two of the currently available pyrethroid‐PBO nets (PermaNet 3.0 and DawaPlus 3.0), only contain PBO on the roof of the netting, exploiting the behavioural patterns of host‐seeking mosquitoes to attempt to reach the net user by approaching from above (Parker 2015), whilst the remaining pyrethroid‐PBO nets contain the synergist on all sides of the net. The amount of PBO contained within the net differs by a factor of 25‐fold. It is not known how the net manufacturers selected the doses of PBO to apply to the netting.
With the currently available data it is not possible to draw any conclusions on which strategy for producing pyrethroid‐PBO nets will prove to be the most effective under field conditions. The optimum PBO:pyrethroid ratio will likely differ depending on the level of resistance in the mosquito and the underpinning resistance mechanisms. Data from experimental hut trials suggest that the PBO component of the pyrethroid‐PBO nets is lost after repeated washing, as the enhanced mortality caused by the synergist nets is not maintained after 20 washes. As yet, no trials on the durability of pyrethroid‐PBO nets under operational conditions have been published. Encouragingly, the only clinical trial of pyrethroid‐PBO nets found that the superior protective efficacy of Olyset Plus compared to standard Olyset nets was maintained over 20 months of use (Protopopoff 2018); this trial is being extended further to establish whether this effect lasts the full duration of an LLIN's intended 36‐month lifespan.
Most of the available data evaluated the performance of pyrethroid‐PBO LLINs against An gambiae s.l., with very limited data available for the second major species complex in Africa, An funestus, and none for other minor vector species. As different mosquito species may differ in their behaviour, and in the strength and underpinning mechanisms of pyrethroid resistance, this represents an important data gap that may have implications for practice in areas where An gambiae complex is not the predominant malaria vector.
Potential biases in the review process
As the addition of PBO to pyrethroid LLINs is only expected to enhance their performance in areas where the mosquitoes are resistant to pyrethroid insecticides, it was important to stratify the results by resistance status. To do this we used the WHO definition of resistance as mosquito populations with less than 90% mortality in a discriminating dose assay (WHO 2016), and then split the resistant populations into three groups, depending on the percentage of mortality observed. Discriminating dose assays provide an estimate of the prevalence of resistance in a population but do not indicate the strength of this resistance or give any indication of the mechanism(s) underpinning this resistance. As PBO works primarily by inhibiting the metabolism of pyrethroids by cytochrome P450s, this synergist is likely to have had most impact in populations where resistance was primarily conferred by elevated P450 activity and further stratification according to resistance mechanism might have proved informative. However, in reality, characterization of resistance in mosquitoes is still primarily performed by bioassays alone and, the relevant contribution of different resistance mechanisms to the phenotype remains unknown. An exception to this is in An funestus, where pyrethroid resistance is almost entirely due to elevated P450 activity (Churcher 2016). Unfortunately only one data set from experimental hut trials conducted where An funestus was the primary vector were made available to us at the time of this review.
Other examples of missing data that may have influenced the results include the absence of data on resistance status in some settings. Three experimental hut trials did not measure resistance at the time of the trial (Moore 2016; N'Guessan 2010; Pennetier 2013). For two of these trials we used proxies for resistance; however, no proxy data were available for An funestus in the Moore 2016 trial and hence we did not include this population in the stratified analysis. Three trials did not share their data with the review authors; this included trials on nets from two of the more recent manufacturers to produce pyrethroid‐PBO nets (N’Guessan 2016; Tungu 2017), which precluded stratified analysis for these net types.
One of the key findings of this trial was the decline in performance of pyrethroid‐PBO nets after washing. However, as discussed above, it is not clear how the standardized washing protocol employed in experimental hut trials of LLINs reflects the actual chemical retention of active ingredients under operational use and hence the policy implications of this remain to be determined.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review of pyrethroid‐PBO nets. An earlier meta‐analysis of experimental hut data indicated that pyrethroid‐PBO nets would have the greatest impact against mosquito populations with intermediate levels of resistance (Churcher 2016). Using transmission models to convert entomological outputs into estimates of public health benefit, the trial noted that the impact of pyrethroid‐PBO nets would vary depending on mosquito species, resistance levels, malaria prevalence, and LLIN usage. The importance of taking these key parameters into account when predicting the public health impact of a switch to pyrethroid‐PBO nets has been somewhat lost in policy documents and operational guidelines which, understandably, seek to provide a simple decision rule to aid net selection. Hence, in the WHO report from the 2017 Evidence Review Group on ‘Conditions for deployment of mosquito nets treated with pyrethroid and piperonyl butoxide', it is recommended that "National malaria control programmes and their partners should consider deployment of pyrethroid‐PBO nets in areas where pyrethroid resistance has been confirmed in the main malaria vectors" (WHO 2017b). In the technical guidelines from one of the major net distributors, the PMI, the conditions for deployment of PBO nets are "moderate levels of pyrethroid resistance (defined as 35‐80% mortality), evidence that PBO restores pyrethroid susceptibility, and moderate to high malaria prevalence" (PMI 2018). The PMI definition of moderate resistance overlaps with our definitions of moderate and low resistance. However in our review, the best evidence for superior efficacy of pyrethroid‐PBO nets is from areas with high resistance (< 30% mortality), and there was very little evidence for improved performance in areas with moderate or low levels of resistance. The differences between these trials may have arisen from the incorporation of a large data set of laboratory bioassays, comparing mosquito mortality with or without pre‐exposure to PBO, in the modelling trial. These laboratory bioassays rely on use of a single discriminating dose and identified multiple trials where highly resistant populations were not impacted by PBO. In the current trial, the mosquito populations included were limited to the sites in which experimental hut trials had been conducted and this may not have fully captured the full diversity of resistance mechanisms in Anopheles mosquitoes. This again highlights the importance of further trials on the influence of resistance mechanisms on the impact of pyrethroid‐PBO LLINs.
Authors' conclusions
Implications for practice.
The findings of this review support the recent WHO policy recommendation that pyrethroid‐piperonyl butoxide (PBO) nets should be considered for deployment in areas where pyrethroid resistance has been confirmed in the main malaria vectors (WHO‐GMP 2017a). Questions remain about the durability of the pyrethroid‐PBO nets. Encouragingly the single clinical trial of pyrethroid‐PBO nets did find that the protective efficacy of these nets lasted at least 21 months and this trial is being extended to see if this effect lasts the full 36 months of the intended lifespan of a long‐lasting insecticidal net (LLIN). The WHO has declared Olyset Plus as the first in class for pyrethroid‐PBO nets and as a result, pyrethroid‐PBO nets from other manufacturers will not be required to generate epidemiological evidence on their efficacy. However, a second clinical trial of pyrethroid‐PBO nets is underway in Uganda; this four‐arm trial is comparing two pyrethroid‐PBO nets (PermaNet 3.0 and Olyset Plus) with two standard LLINs (PermaNet 2.0 and Olyset).
When evaluating these trials it is important to remember that the PBO is an additive to the nets, intended to increase their efficacy against pyrethroid‐resistant mosquito populations. There is no evidence to suggest that pyrethroid‐PBO nets are less effective than standard LLINs at inducing mosquito mortality under any settings. For personal protection, blood‐feeding rates are similarly decreased under all resistance scenarios when unwashed PBO nets are used, although this has not been shown for washed nets in low‐resistance or susceptible areas (low‐certainty evidence). Hence if pyrethroid‐PBO nets perform as well, or better, than standard LLINs, the decision on whether to switch to nets incorporating the synergist is largely a question of economics. With fixed budgets there is a risk that the target of universal coverage of LLINs may be harder to reach if more expensive pyrethroid‐PBO nets are deployed. Indeed, the WHO clearly states that countries should only consider deploying pyrethroid‐PBO nets in situations where coverage with standard vector‐control interventions is not reduced (WHO‐GMP 2017c). Trials of the cost effectiveness of pyrethroid‐PBO nets have not yet been possible due to the uncertainties over the price differential between pyrethroid‐PBO nets and LLINs.
Implications for research.
Experimental hut trials, simultaneously comparing different pyrethroid‐PBO nets in areas where mosquitoes have high levels of pyrethroid resistance levels are needed to demonstrate equivalency and inform procurement decisions, particularly given the very different approaches used to incorporate PBO into LLINs employed by the different manufacturers. The issue of durability of bioactive levels of the synergist on the nets also needs further study; current WHO protocols for measuring LLIN durability will need adjusting to utilize pyrethroid‐resistant colonies of mosquitoes so that the impact of PBO, and not just the insecticide, can be measured over the net's intended lifespan. The issue of the value of entomological endpoints to estimate the public health value of new types of net remains contentious (Killeen 2018; WHO‐GMP 2017c). Performing experimental hut trials alongside future randomized controlled trials of nets containing synergists, or other novel active ingredients, would help resolve this issue.
In relation to reporting of trial results, study authors need to record the level of resistance in the local mosquito population at the time of the trial and include this when reporting the results. Data on resistance mechanisms would also be of value to understand more about how this influences the performance of pyrethroid‐PBO nets.
What's new
| Date | Event | Description |
|---|---|---|
| 6 June 2019 | Amended | Amended Abstract, Authors' conclusions section: from "reduce mosquito mortality and blood feeding rates" to "increase mosquito mortality and reduce blood feeding rates". |
Acknowledgements
The Academic Editor of this review is Dr Hellen Gelband.
We thank Vittoria Lutje, Cochrane Infectious Diseases Group (CIDG) Information Specialist, for her assistance in conducting the literature search and Paul Garner, CIDG Co‐ordinating Editor, for his guidance during the protocol preparation process.
Leslie Choi and Marty Richardson are supported by the Research, Evidence and Development Initiative (READ‐It) project. READ‐It and the editorial base of the Cochrane Infectious Diseases Group are funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
This work was partly supported through a grant from the Global Malaria Programme, World Health Organization.
Appendices
Appendix 1. Detailed search strategies
Cochrane Library
Description:
#1 "piperonyl butoxide" or PBO:ti,ab,kw (Word variations have been searched)
#2 MeSH descriptor: [Piperonyl Butoxide] explode all trees
#3 #1 or #2
#4 Net* or bednet* or hammock* or curtain* or ITN* or LLIN* or "Insecticide‐Treated Bednet*" or "Insecticide‐Treated net*"
#5 Olyset* or PermaNet* or Veeralin
#6 MeSH descriptor: [Insecticide‐Treated Bednets] explode all trees
#7 #4 or #5 or #6
#8 #3 and #7
MEDLINE (PubMed)
| Query | |
| #1 | Search "Piperonyl Butoxide"[Mesh] |
| #2 | Search "piperonyl butoxide" or PBO Field: Title/Abstract |
| #3 | Search Olyset* or PermaNet* or Veeralin Field: Title/Abstract |
| #4 | Search "Insecticide‐Treated Bednets"[Mesh] Field: Title/Abstract |
| #5 | Search Nets OR bednet* OR hammock* OR curtain* OR ITN* OR LLIN* or “Insecticide‐Treated Bednet*” or “Insecticide‐Treated net*” Field: Title/Abstract |
| #6 | Search ((#5) OR #4) OR #3 Field: Title/Abstract |
| #7 | Search (#2) OR #1 |
| #8 | Search (#7) AND #6 |
Embase (OVID)
1 "piperonyl butoxide".mp.
2 piperonyl butoxide/
3 PBO.mp.
4 placebo/ or placebo.mp.
5 3 not 4
6 1 or 2 or 5
7 (Net* or bednet* or hammock* or curtain* or ITN* or LLIN* or "Insecticide‐Treated Bednet*" or "Insecticide‐Treated net*").mp.
8 (Olyset* or PermaNet* or Veeralin).mp.
9 insecticide treated net/
10 7 or 8 or 9
11 6 and 10
Web of ScienceTM Core Collection
| Set | |
| # 8 | #7 AND #6 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 7 |
TOPIC: (malaria or mosquito* or pyrethroid* or insect* or huts or insecticide*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 6 | #5 AND #4 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 5 |
TOPIC: (Net* OR bednet* OR insecticide treated net/ OR ITN* OR LLIN* or “Insecticide‐Treated Bednet*” or “Insecticide‐Treated net*”) ORTOPIC: (Olyset* or PermaNet* or Veeralin) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 4 | #3 OR #1 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 3 |
TOPIC: (PBO) NOTTOPIC: (placebo) Refined by: RESEARCH AREAS: ( CHEMISTRY ) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 2 |
TOPIC: (PBO) NOTTOPIC: (placebo) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 1 |
TOPIC: ("Piperonyl Butoxide") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
CABI: CAB Abstracts®
| Set | |
| # 3 | #2 AND #1 Indexes=CAB Abstracts Timespan=All years |
| # 2 |
TOPIC: (Net* OR bednet* OR hammock* OR curtain* OR ITN* OR LLIN* or “Insecticide‐Treated Bednet*” or “Insecticide‐Treated net*”) ORTOPIC: (Olyset* or PermaNet* or Veeralin) Indexes=CAB Abstracts Timespan=All years |
| # 1 |
TOPIC: (PBO or "Piperonyl Butoxide") Indexes=CAB Abstracts Timespan=All years |
ClinicalTrials.gov and WHO ICTRP
piperonyl butoxide and malaria
Appendix 2. Study characteristics extraction form
Table 2.1 Trial characteristics of the included cone bioassays
| TrialID | Trialname | Mosquito species (strain/origin) | Resistance level | Resistance status | Mosquito age | Blood‐fed status | Intervention | Washing | Measured outcome; mosquito mortality |
Table 2.2 Trial characteristics of the included ball bioassays
| TrialID | Trialname | Mosquito species (strain/origin) | Resistance level | Resistance status | Mosquito age | Blood‐fed status | Intervention | Washing | Measured outcome; mosquito mortality |
Table 2.3 Trial characteristics of the included tunnel tests
| TrialID | Trialname | Mosquito species (strain/origin) | Resistance level | Resistance status | Mosquito age | Blood‐fed status | Intervention | Washing | Measured outcome | |||
| KD | M | BF | NPTN | |||||||||
Abbreviations: KD: knock‐down; M: mortality; BF; blood feeding; NPTN: not passed though net.
Table 2.4 Trial characteristics of the included experimental hut trials
| TrialID | Trialname | Triallocation | Mosquito species (strain/origin) | Resistance level | Resistance status | Trialstart/end date | Intervention | Net washed | Net holed | Measured outcome | |||
| M | BF | D | E | ||||||||||
Abbreviations: M: mortality; BF; blood feeding; D: deterrence; E: exophily.
Table 2.5 Trial characteristics of the included village trials
| TrialID | Trialname | Triallocation | Mosquito species (strain/origin) | Resistance level | Resistance status | Trialstart/end date | Intervention | Net washed | Net holed | Measured outcome | ||||||
| M | BF | SR | MD | PR | PP | CMC | ||||||||||
Abbreviations: M: mortality; BF: blood feeding; SR: sporozoite rate; MD: mosquito density; PR: parity rate; PP: parasite presence; CMC: clinical malaria confirmation.
Appendix 3. Data extraction form
Table 3.1 Data extracted from laboratory bioassays.
| TrialID | Trialname | Net type | Net washed | Mosquito species | Resistance level | Resistance status | Total mosquito | Dead | Mosquito mortality (%) | BF | BF (%) | NPTN | NPTN (%) |
Abbreviations: BF: blood feeding; NPTN: not passed through net.
Table 3.2 Data extracted from experimental hut trials
| TrialID | Trialname | Net type | Net washed | Net holed | Mosquito species | Resistance level | Resistance status | Total mosquitoes | Dead | Mosquito mortality (%) | BF | BF (%) | BFI | Number of mosquitoes deterred | Deterrence (%) | Exit trap | Exophily (%) | Total number of people (N) |
Abbreviations: BF: blood feeding; BFI: blood feeding inhibition; N: number of people.
Table 3.3 Data extracted from village trials
| TrialID | Trialname | Net type | Net washed | Net holed | Mosquito species | Resistance level | Resistance status | Total mosquitoes | Dead | Mosquito mortality (%) | BF | BF (%) | BFI | Sporozoite (%) | Mosquito density (%) | Parity (%) | Total number of people (N) |
PP (%) |
CMC (%) |
Abbreviations: BF: blood feeding; PP: parasite prevalence; CMC: clinical malaria confirmation; N: number of people.
Appendix 4. ‘Risk of bias' assessment form
Table 4.1 ‘Risk of bias' assessment for laboratory bioassays
| TrialID | Trialname | Comparability of mosquitoes in LLIN and LLIN + PBO groups | Observers blinded | Incomplete outcome data | Raw data reported for LLIN and LLIN + PBO groups | Authors' conflicting interest |
Abbreviations: LLIN: long‐lasting insecticidal nets; PBO: piperonyl butoxide.
Table 4.2 ‘Risk of bias' assessment for experimental hut trials
| TrialID | Trialname | Comparability of mosquitoes in LLIN and LLIN+PBO groups | Collectors blinded | Sleepers blinded | Sleeper bias | Treatment allocation | Treatment rotation | Standardized hut design | Hut cleaning between treatments | Incomplete outcome data | Raw data reported for both treatment groups | Authors' conflicting interest |
Table 4.3 ‘Risk of bias' assessment for village trials
| TrialID | Trialname | Comparability of mosquitoes in LLIN and LLIN + PBO households | Collectors blinded | Household blinded | Allocation of treatments | Incomplete outcome data | Raw data reported for both groups | Authors' conflicting interest |
Abbreviations: LLIN: long‐lasting insecticidal nets; PBO: piperonyl butoxide.
Appendix 5. ‘Risk of bias' assessment: laboratory bioassays
| Risk of bias component | Low | Unclear | High |
| Mosquito group comparability | Same strain or population of mosquitoes | No or unclear information reported | Different strain or population of mosquitoes |
| Observers blinded | Outcomes assessed blinded | No or unclear information reported If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
Outcomes assessed not blinded, and this is likely to influence the results If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
| Incomplete outcome data addressed | No or low missing data, reason for missing data is unlikely to be related to the true outcome | No or unclear information reported | High missing data, reason for missing data is likely to be related to the true outcome |
| Raw data reported | Raw data reported | No or unclear information reported | Raw data not reported |
| Conflicting interests | No conflict of interest is stated | No or unclear information reported | Conflict of interest stated |
Appendix 6. ‘Risk of bias' assessment: experimental hut trials
| Risk of bias component | Low | Unclear | High |
| Mosquito group comparability | Huts accessible to the same mosquito population | No or unclear information reported | Huts not accessible to the same mosquito population |
| Collectors blinded | Outcomes assessed blinded | No or unclear information reported If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
Outcomes assessed not blinded, and this is likely to influence the results If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
| Sleepers blinded | Outcomes assessed blinded | No or unclear information reported If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk |
Outcomes assessed not blinded, and this is likely to influence the results. If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk |
| Sleeper bias | Sleepers were rotated between huts using a Latin square design | No or unclear information reported | Sleepers not rotated between huts |
| Treatment allocation | Treatments randomized Treatments not‐randomized however equal attractiveness demonstrated |
No or unclear information reported | Treatments not randomized, and equal attractiveness not demonstrated |
| Treatment rotation | Treatments rotated through huts using a Latin square design | No or unclear information reported | Treatments not rotated |
| Standardized hut design | Huts of West‐ or East‐African design | No or unclear information reported | Huts of non‐standardized design |
| Cleaning | Huts cleaned between treatments | No or unclear information reported | Huts not cleaned between treatments |
| Incomplete outcome data addressed | No or low missing data, reason for missing data is unlikely to be related to the true outcome | No or unclear information reported | High missing data, reason for missing data is likely to be related to the true outcome |
| Raw data reported | Raw data reported | No or unclear information reported | Raw data not reported |
| Conflicting interests | No conflict of interest is stated | No or unclear information reported | Conflict of interest stated |
Appendix 7. ‘Risk of bias' assessment: village trials
| Risk of bias component | Low | Unclear | High |
| Recruitment bias | No participants recruited after clusters randomized | No or unclear information reported Recruitment bias not applicable to trial design as it is related to human participants |
Paricipants recruited to trial after clusters randomized |
| Mosquito group comparability | Mosquito populations comparable | No or unclear information reported | Mosquito populations comparable |
| Collectors blinded | Outcomes assessed blinded | No or unclear information reported Outcomes assessed not blinded, but this is unlikely to influence the results |
Outcomes assessed not blinded, and this is likely to influence the results |
| Household blinded | Outcomes assessed blinded | No or unclear information reported If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
Outcomes assessed not blinded, and this is likely to influence the results If outcomes assessed were not blinded, but this is unlikely to influence the results, we will judge this to be low risk. |
| Treatment allocation | Treatments randomized | No or unclear information reported | Treatments not randomized |
| Allocation concealment | Allocation concealment procedures were adhered to. | No or unclear information reported Allocation concealment procedures where not adhered to, however this is unlikely to affect the results. |
Allocation procedures were not adhered to and this is likely to have affected the results. |
| Incomplete outcome data addressed | No or low missing data, reason for missing data is unlikely to be related to the true outcome | No or unclear information reported | High missing data, reason for missing data is likely to be related to the true outcome |
| Raw data reported | Raw data reported | No or unclear information reported | Raw data not reported |
| Clusters lost to follow‐up | No complete clusters lost from trial | No or unclear information as to whether clusters were lost from trial | At least one cluster lost from trial |
| Selective reporting | No selective reporting, all measured outcomes reported in results | No or unclear information on whether all measured outcomes were reported in results | Selective reporting; not all measured outcomes were reported in results |
| Correct statistical methods; adjusted for clustering | Clustering was taken into account and statistical methods adjusted for clustering | No or unclear information as to whether clustering was taken into account for statistical methods. | Trial did not take clustering into account for statistical methods |
| Conflicting interests | No conflict of interest is stated | No or unclear information reported | Conflict of interest stated |
Data and analyses
Comparison 1. Pyrethroid‐PBO nets versus LLINs: laboratory bioassay (cone trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mosquito mortality | 2 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.06 [4.15, 8.84] |
Comparison 2. Commercial pyrethroid‐PBO nets versus commercial LLINs: hut trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mosquito mortality (pooled) hut/night | 8 | 14334 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.20, 1.54] |
| 1.1 Unwashed | 8 | 7742 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.21, 1.96] |
| 1.2 Washed | 7 | 6592 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.31] |
| 2 Mosquito blood‐feeding success (pooled) hut/night | 7 | 11070 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.67, 0.88] |
| 2.1 Unwashed | 7 | 6355 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.54, 0.79] |
| 2.2 Washed | 6 | 4715 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 3 Mosquito exophily (pooled) hut/night | 8 | 11933 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.06] |
| 3.1 Unwashed | 8 | 6793 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| 3.2 Washed | 7 | 5140 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 4 Mosquito mortality (high resistance) hut/night | 5 | 7997 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.34, 1.86] |
| 4.1 Unwashed | 5 | 4896 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.60, 2.11] |
| 4.2 Washed | 4 | 3101 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.88, 1.63] |
| 5 Mosquito blood‐feeding success (high resistance) hut/night | 4 | 7134 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.76] |
| 5.1 Unwashed | 4 | 4458 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.50, 0.71] |
| 5.2 Washed | 3 | 2676 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.92] |
| 6 Mosquito mortality (moderate resistance) hut/night | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.40] |
| 6.1 Unwashed | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.88, 1.54] |
| 6.2 Washed | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.54] |
| 7 Mosquito blood‐feeding success (moderate resistance) hut/night | 1 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.06] |
| 7.1 Unwashed | 1 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 7.2 Washed | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.13] |
| 8 Mosquito mortality (low resistance) hut/night | 1 | 1224 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.02, 1.25] |
| 8.1 Unwashed | 1 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.05, 1.16] |
| 8.2 Washed | 1 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.83, 1.63] |
| 9 Mosquito blood‐feeding success (low resistance) hut/night | 1 | 1224 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.85, 2.41] |
| 9.1 Unwashed | 1 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.06, 7.37] |
| 9.2 Washed | 1 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.89, 2.54] |
| 10 Mosquito mortality (susceptible) hut/night | 2 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 10.1 Unwashed | 2 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.64, 2.26] |
| 10.2 Washed | 2 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.25] |
| 11 Mosquito blood‐feeding success (susceptible) hut/night | 2 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.89] |
| 11.1 Unwashed | 2 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.12, 2.22] |
| 11.2 Washed | 2 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.82, 1.91] |
| 12 Mosquito mortality (high resistance/Permanet) hut/night | 3 | 2806 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.26, 2.01] |
| 12.1 Not Washed | 3 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.56, 2.10] |
| 12.2 Washed | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.61, 2.28] |
| 13 Mosquito blood‐feeding success (high resistance/Permanet) hut/night | 2 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.45, 0.76] |
| 13.1 Unwashed | 2 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.69] |
| 13.2 Washed | 1 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.93] |
| 14 Mosquito mortality (high resistance/Olyset) hut/night | 2 | 1410 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.51, 1.97] |
| 14.1 Unwashed | 2 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.48, 1.99] |
| 14.2 Washed | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.25, 2.61] |
| 15 Mosquito blood‐feeding success (high resistance/Olyset) hut/night | 2 | 1470 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.98] |
| 15.1 Unwashed | 2 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.18] |
| 15.2 Washed | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.93] |
Comparison 3. Commercial pyrethroid‐PBO nets versus commercial LLINs: village trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prevalence of malaria | 1 | Odds Ratio (Fixed, 95% CI) | 0.40 [0.20, 0.80] | |
| 2 Mosquito sporozoite‐positive (adjusted ICC 0.1) | 4 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.24, 2.75] |
| 3 Mosquito parous (adjusted ICC 0.1) | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.13] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Awolola 2014.
| Methods | Village trial | |
| Participants | Ilara ‐ An gambiae (100% S‐form) Irolu ‐ 95% An gambiae (100% S‐form), 4.5% An arabiensis Ijesa ‐ 98.1% An gambiae (80% S‐form, 19% M‐form), An arabiensis (1.6%) | |
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Mosquito mortality, blood feeding, sporozoite rate, mosquito density, parity rate | |
| Mosquito resistance status | Ilara ‐ resistant ‐ low (deltamethrin, 72.5% mortality, N = 120) Irolu ‐ resistant ‐ low (deltamethrin, 62.5% mortality, N = 120) Ijesa ‐ resistant ‐ low (deltamethrin, 66.7% mortality, N = 120) | |
| Net treatment | Nets unholed and unwashed | |
| Location(s) | Ilara, Nigeria ‐ untreated net Irolu, Nigeria ‐ PermaNet 2.0 Ijesa, Nigera ‐ PermaNet 3.0 | |
| Notes | Trial conducted: March 2012‐March 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias | Low risk | Recruiment bias is related to human participants and so not applicable to this study. |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Unclear risk | Mosquito species composition varied slightly pre‐ and post‐trial between the treatment villages. However, resistance level was the same. |
| Collectors blinded | High risk | Not stated if collectors where blinded, therefore judged as high risk as this is likely to impact searching effort. |
| Household blinded | Low risk | Unclear if households were blinded – not stated in the publication. We judged this as low as this is unlikely to affect the outcome. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Villages were randomly assigned to treatment arms |
| Allocation concealment (selection bias) | Low risk | Allocation concealment procedures were not adhered to, however this is unlikely to affect the results |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Clusters lost to follow‐up | Low risk | No clusters lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measured outcomes appear to be reported |
| Correct statistical methods; adjusted for clustering | High risk | Study did not take clustering into account for statistical methods |
| Trial authors' conflicting interest | Low risk | The trial authors declared no conflicting interests, however the study was funded by Vestergaard (net manufacturers). Views and findings in the publication are stated to be those of the trial authors |
Bayili 2017.
| Methods | Experimental hut trial | |
| Participants | An coluzzii | |
| Interventions | Control: LLIN, DawaPlus 2.0 Intervention: LLIN: DawaPlus 3.0, DawaPlus 4.0 |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Resistant ‐ high (6% mortality, N = 98) | |
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Vallée du Kou, Burkina Faso | |
| Notes | Trial conducted: August 2016‐October 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | The hut trial was conducted in the same area, therefore characteristics are similar |
| Collectors blinded | Unclear risk | Paper does not state if collectors were blinded |
| Sleepers blinded | Unclear risk | Paper does not state if sleepers were blinded |
| Sleeper bias | Low risk | Sleepers were rotated between hut following a Latin square design |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were not randomly allocated to huts however the trial completed a full rotation through the huts |
| Treatment rotation | Low risk | Treatments were rotated between hut following a Latin square design + 2 weeks |
| Standardized hut design | Low risk | Hut were built previously using standard West‐African design |
| Hut cleaning between treatments | Unclear risk | Does not state if huts were cleaned between treatments |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data was reported |
| Trial authors' conflicting interest | Low risk | The authors declare no conflicting interest in the WHOPES report. |
Cisse 2017.
| Methods | Village trial | |
| Participants | An gambiae s. s. | |
| Interventions | Control: LLIN, Olyset Net & PermaNet 2.0 Intervention: LLIN, Olyset Plus & PermaNet 3.0 |
|
| Outcomes | Sporozoite rate, mosquito density, parity rate | |
| Mosquito resistance status | Olyset Net villages ‐ resistance ‐ high (1% mortality, N = 305) Olyset Plus villages ‐ resistance ‐ high (2% mortality, N = 411) PermaNet 2.0 villages ‐ resistance ‐ high (29% mortality, N = 410) PermaNet 3.0 villages ‐ resistance ‐ moderate (38% mortality, N = 408) | |
| Net treatment | Nets unholed and unwashed | |
| Location(s) | Sikasso region, Mali PermaNet 2.0 villages ‐ Beko East, Dalabani, Berila, Dierila PermaNet 3.0 villages ‐ Beko West, Farabacoura East, Kola Djokada, Tieblembougou Olyset Net villages ‐ Karako, Geleba 2, Toula East, Toula West Olyset Plus villages ‐ Dialake, Farabacoura West, Deneklin, Faradjele |
|
| Notes | Trial conducted: January 2014‐January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias | Low risk | Recruiment bias is related to human participants and so not applicable to this study |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Unclear risk | Mosquito species composition constant between villages, however resistance level varies slightly. |
| Collectors blinded | High risk | Not stated if collectors where blinded therefore judged as high risk as this is likely to affect searching effort. |
| Household blinded | Low risk | Unclear if households were blinded – not stated in the publication. We judged this as low as this is unlikely to affect the outcome |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Villages were randomly assigned to treatment arms. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment procedures were not adhered to, however this is unlikely to affect the results. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported |
| Clusters lost to follow‐up | Low risk | No clusters lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measured outcomes appear to be reported. |
| Correct statistical methods; adjusted for clustering | High risk | Study did not take clustering into account for statistical methods. |
| Trial authors' conflicting interest | Low risk | The trial authors have no competing interests |
Corbel 2010.
| Methods | Experimental hut trial | |
| Participants | Vallée du Kou, Burkina Faso ‐ 100% An gambiae: M‐form (15%), S‐form (85%) Malanville, Benin ‐ 95% An gambiae: M‐form (100%), 5% An arabiensis Pitoa, Cameroon ‐ 5% An gambiae: S‐form (100%), 95% An arabiensis |
|
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Vallée du Kou, Burkina Faso ‐ resistant – high (deltamethrin, 23% mortality, N = 100) Malanville, Benin ‐ resistant – low (deltamethrin, 85% mortality, N = 100) Pitoa, Cameroon ‐ resistant – low (deltamethrin, 70% mortality, N = 100) |
|
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Vallée du Kou, Burkina Faso Malanville, Benin Pitoa, Cameroon |
|
| Notes | Trial conducted: Vallée du Kou, Burkina Faso ‐ September 2007 to November 2007 Malanville, Benin ‐ July 2008 to September 2008 Pitoa, Cameroon ‐ July 2008 to September 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were randomly allocated to huts. |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design. |
| Hut cleaning between treatments | Unclear risk | Unclear if huts were cleaned between treatments – not stated in publication |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete outcome data |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Trial authors' conflicting interest | Low risk | The trial authors have no competing interests |
Darriet 2011.
| Methods | Cone assay | |
| Participants | 2‐5‐day‐old female unfed An gambiae VKPR laboratory strain (originated from Kou Valley, Burkina‐Faso and maintained for > 15 years under laboratory conditions) | |
| Interventions | Control: ITN treated with deltamethrin (25 mg/m2) Intervention: ITN treated with deltamethrin (25 mg/m2) & PBO (222.24 mg/m2) |
|
| Outcomes | Mosquito mortality | |
| Mosquito resistance status | Resistant colony, unclassified | |
| Net treatment | Nets unwashed | |
| Location(s) | N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Same mosquito strain used for all treatment groups |
| Were the study observers blinded to the allocated intervention | Unclear risk | Unclear if observers were blinded – not stated in the publication, but also not common practice. We judged this as low as this is unlikely to impact on the outcome. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | The resistance status of the Anopheles gambiae VKPR lab strain is not stated in the publication. |
| Trial authors' conflicting interest | Low risk | The trial authors do not state any conflicting interests |
Darriet 2013.
| Methods | Cone assay | |
| Participants | 2‐5‐day‐old female unfed An gambiae VKPR laboratory strain (originated from Kou Valley, Burkina‐Faso and maintained for > 15 years under laboratory conditions) | |
| Interventions | Control: ITN treated with deltamethrin (25 mg/m2) Intervention: ITN treated with deltamethrin (25 mg/m2) & PBO (222.24 mg/m2) |
|
| Outcomes | Mosquito mortality | |
| Mosquito resistance status | Resistant colony, unclassified | |
| Net treatment | N/A | |
| Location(s) | N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Same mosquito strain used for all treatment groups |
| Were the study observers blinded to the allocated intervention | Unclear risk | Unclear if observers were blinded – not stated in the publication, but also not common practice. We judged this as low as this is unlikely to impact on the outcome. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | The resistance status of the Anopheles gambiae VKPR lab strain is not stated in the publication. |
| Trial authors' conflicting interest | Low risk | The trial authors do not state any conflicting interests. |
Koudou 2011.
| Methods | Experimental hut trial | |
| Participants | An gambiae s.s. | |
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Mosquito mortality, deterrence, exophily | |
| Mosquito resistance status | Resistant ‐ high (deltamethrin, 10.6% mortality, N = 80 min) | |
| Net treatment | Nets not holed, nets unwashed and washed (x 20) | |
| Location(s) | Yaokoffikro, Côte d'Ivoire | |
| Notes | Trial conducted: April 2009‐July 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were not randomly allocated to the huts. However, results from trials performed before this trial showed no significant difference in attractiveness of the different huts |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design |
| Hut cleaning between treatments | Low risk | All huts were cleaned between treatments |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported |
| Trial authors' conflicting interest | Low risk | The trial authors declared there were no conflicting interests. |
Moore 2016.
| Methods | Experimental hut trial | |
| Participants | An arabiensis (100%), An funestus group (95% s.s.) | |
| Interventions | Control: LLIN, MAGNet LN Intervention: LLIN, Veeralin LN |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status |
An arabiensis ‐ susceptible (alphacypermethrin, 100% mortality, N = 97) An funestus ‐ unclassified |
|
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Ifakara, Tanzania | |
| Notes | Although additional data provided showed resistance to deltamethrin and permethrin in An gambaie s.l. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | The hut trial was conducted in the same area, therefore characteristics are similar |
| Collectors blinded | Unclear risk | Paper does not state if collectors were blinded. |
| Sleepers blinded | Unclear risk | Paper does not state if sleepers were blinded. |
| Sleeper bias | Low risk | Sleepers were rotated between hut following a Latin square design. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were not randomly allocated to huts however the trial completed a full rotation through the huts. |
| Treatment rotation | Low risk | Treatments were rotated between hut following a Latin square design. |
| Standardized hut design | Low risk | The study used the standard design of the Ifakara experimental huts. |
| Hut cleaning between treatments | Unclear risk | The paper does not state if huts were cleared between treatments. |
| Were incomplete outcome data adequately addressed | Low risk | No incomplete outcome data |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | No missing outcome data |
| Trial authors' conflicting interest | Low risk | The trial authors declared they received prescribed standard fees from Vestergaard Frandsen for evaluating their pesticide products, however this is standard practice. |
Mzilahowa 2014.
| Methods | Village trial | |
| Participants | An gambiae s. l., An funestus group | |
| Interventions | Control: LLIN, Olyset Net & PermaNet 2.0 Intervention: LLIN, Olyset Plus & PermaNet 3.0 |
|
| Outcomes | Mosquito density, parity rate | |
| Mosquito resistance status |
An funestus (Balaka district) Permethrin ‐ resistant ‐ moderate (55.5% mortality, N = unknown) Deltamethrin ‐ resistant ‐ high (14.9% mortality, N = unknown) An gambiae (Balaka district) Permethrin ‐ resistant ‐ low (84.4% mortality, N = unknown) (Machinga district) Deltamethrin ‐ resistant ‐ moderate (54.5% mortality, N = unknown) |
|
| Net treatment | Nets unholed and unwashed | |
| Location(s) | Balaka district, Malawi (12 villages) | |
| Notes | Trial conducted: December 2012‐June 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias | Low risk | Recruiment bias is related to human participants and so not applicable to this study. |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | High risk | Mosquito species composition and resistance status are not recorded per village. Village names are not provided in study, instead villages are grouped by treatment type. |
| Collectors blinded | High risk | Not stated if collectors were blinded, therefore judged as high risk as this is likely to affect searching effort. |
| Household blinded | Low risk | Unclear if households were blinded – not stated in the publication. We judged this as low as this is unlikely to affect the outcome. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Villages were randomly assigned to treatment arms. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment procedures were not adhered to, however this is unlikely to affect the results. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported |
| Clusters lost to follow‐up | Low risk | No clusters lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes appear to be reported |
| Correct statistical methods; adjusted for clustering | High risk | Study did not take clustering into account when performing statistical methods |
| Trial authors' conflicting interest | Unclear risk | There is no information on trial authors' possible conflicting interests provided. |
N'Guessan 2010.
| Methods | Experimental hut trial | |
| Participants | An gambiae | |
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Proxy data. Adjara, Benin: resistant ‐ moderate (deltamethrin, 50% mortality, N = 56) (Aïzoun 2013) | |
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Akron, Benin | |
| Notes | Trial conducted: October 2008‐January 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were randomly allocated to huts |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design. |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design. |
| Hut cleaning between treatments | Low risk | All huts were cleaned between treatments. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported |
| Trial authors' conflicting interest | Unclear risk | The trial was sponsored by Vestergaard (net manufacturers), who also commented on the manuscript |
Pennetier 2013.
| Methods | Experimental hut trial | |
| Participants | 95% An gambiae: M‐form (100%), 5% An arabiensis (Corbel 2010) | |
| Interventions | Control: LLIN, Olyset Net Intervention: LLIN, Olyset Plus |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Proxy data. Resistant ‐ high (permethrin, 22% mortality, N = 100) (Djègbè 2011) | |
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Malanville, Benin | |
| Notes | Trial conducted: September 2011‐December 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were not randomized to huts, but instead were rotated fully between all of the huts using a Latin square design. |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design. |
| Hut cleaning between treatments | Low risk | All huts were cleaned between treatments. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Trial authors' conflicting interest | Low risk | Funders of the trial stated that they had no part in data collection, data analysis or manuscript preparation |
Protopopoff 2018.
| Methods | Cluster‐randomized controlled village trial | |
| Participants | 3966 children analysed (21 months after intervention) aged 6 months‐14 years (excluding the severely ill), Anopheles species (pooled). Total core cluster population area ranged from 14,845 to 16,358. | |
| Interventions | Control: LLIN, Olyset Net Intervention: LLIN, Olyset Plus |
|
| Outcomes | Malaria infection prevalence, sporozoite rate, mosquito density | |
| Mosquito resistance status | Resistance ‐ high (17.8% mortality, N = 107) | |
| Net treatment | Nets unholed and unwashed | |
| Location(s) | Muleba District, Tanzania | |
| Notes | Trial conducted: March 2014‐December 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias | Low risk | No participants recruited after clusters had been randomized |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Unclear risk | Resistance level was only available for the whole district, not at village level. |
| Collectors blinded | Low risk | Field workers were masked to net treatment. |
| Household blinded | Low risk | Inhabitants were masked to net treatment |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Restricted randomization was used to allocate clusters to the study groups. |
| Allocation concealment (selection bias) | Low risk | Restricted randomization was used to allocate treatments to clusters |
| Were incomplete outcome data adequately addressed | Low risk | No incomplete outcome data |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | No missing outcome data |
| Clusters lost to follow‐up | Low risk | No clusters lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measured outcomes appear to be reported |
| Correct statistical methods; adjusted for clustering | Low risk | Clustering was taken into account and adjusted for during statistical analysis |
| Trial authors' conflicting interest | Low risk | The trial authors declared no conflicting interests. |
Stiles‐Ocran 2013.
| Methods | Village trial | |
| Participants | An gambiae | |
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Sporozoite rate, mosquito density, parity rate | |
| Mosquito resistance status | Futa ‐ resistant ‐ moderate (33.3% mortality, N = 96) Abrabra‐ resistant ‐ moderate (43.7% mortality, N = 126) Kunkumso ‐ resistant ‐ high (28.4% mortality, N = 109) Anyinabrim ‐ resistant ‐ moderate (53.2% mortality, N = 109) Wenchi ‐ resistant ‐ low (61.9% mortality, N =126) | |
| Net treatment | Nets unholed and unwashed | |
| Location(s) | Futa, Ghana ‐ no net control Abrabra, Ghana ‐ PermaNet 2.0 Kunkumso, Ghana ‐ PermaNet 2.0 Anyinabrim, Ghana ‐ PermaNet 3.0 Wench, Ghana ‐ PermaNet 3.0 | |
| Notes | Trial conducted: November 2010‐August 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias | Low risk | Recruiment bias is related to human participants and so not applicable to this study. |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Unclear risk | Mosquito species composition varied slightly. Resistance level varies between villages. Data are, however, provided pre‐ and post‐trial. |
| Collectors blinded | High risk | Not stated if collectors were blinded, therefore judged as high risk as this is likely to affect searching effort. |
| Household blinded | Low risk | Unclear if households were blinded – not stated in the publication. We judged this as low as this is unlikely to impact on the outcome. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Villages were randomly assigned to treatment arms. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment procedures were not adhered to, however this is unlikely to affect the results. |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Clusters lost to follow‐up | Low risk | No clusters lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measured outcomes appear to be reported. |
| Correct statistical methods; adjusted for clustering | High risk | Study did not take clustering into account for statistical methods |
| Trial authors' conflicting interest | Unclear risk | Study data were collected for use in Vestergaard PermaNet 3.0 product dossier |
Toé 2018.
| Methods | Experimental hut trial | |
| Participants | An coluzzii | |
| Interventions | Control: LLIN, PermaNet 2.0, Olyset Net Intervention: LLIN, PermaNet 3.0, Olyset Plus |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Vallée du Kou 5 ‐ resistant – high (deltamethrin, 2.5% mortality, N = 163; permethrin, 5% mortality, N = 153) Tengrela ‐ resistant – high (deltamethrin, 34% mortality, N = 85; permethrin, 14% mortality, N = 101) |
|
| Net treatment | Nets holed, nets unwashed | |
| Location(s) | Vallée du Kou 5, Burkina Faso Tengrela, Burkina Faso |
|
| Notes | Trial conducted: September 2014‐October 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were not randomized to huts, but instead were rotated fully between all of the huts using a Latin square design. |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design. |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design. |
| Hut cleaning between treatments | Unclear risk | Unclear if huts were cleaned between treatments – not stated in the publication |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Trial authors' conflicting interest | Low risk | The trial authors have no competing interests. |
Tungu 2010.
| Methods | Experimental hut trial | |
| Participants | An gambiae | |
| Interventions | Control: LLIN, PermaNet 2.0 Intervention: LLIN, PermaNet 3.0 |
|
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily | |
| Mosquito resistance status | Susceptible (deltamethrin, 100% mortality, N = not stated) | |
| Net treatment | Nets holed, nets unwashed and washed (x 20) | |
| Location(s) | Zeneti, Muheza, Tanzania | |
| Notes | Trial conducted: July 2008‐October 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were the mosquitoes in LLIN and LLIN + PBO groups comparable | Low risk | Huts situated in the same area – mosquito characteristics will be the same |
| Collectors blinded | Unclear risk | Unclear if collectors blinded – not stated in publication |
| Sleepers blinded | Unclear risk | Unclear if sleeper blinded – not stated in publication |
| Sleeper bias | Low risk | Sleepers were rotated between huts using a Latin square design. |
| Treatment allocation (was the treatment allocation sequence randomly/adequately generated | Low risk | Treatments were randomly allocated to huts. |
| Treatment rotation | Low risk | Treatments were rotated between huts using a Latin square design. |
| Standardized hut design | Low risk | Huts were built in a standard West‐African design. |
| Hut cleaning between treatments | Low risk | All huts were cleaned between treatments |
| Were incomplete outcome data adequately addressed | Low risk | There were no incomplete data. |
| Were the raw data reported for LLIN and LLIN + PBO groups | Low risk | All necessary data were reported. |
| Trial authors' conflicting interest | Low risk | The trial authors have no competing interests. |
Abbreviations: An arabiensis:Anopheles arabiensis;An coluzzii:Anopheles coluzzii;An funestus: Anopheles funestus;An gambiae: Anopheles gambiae; ITN: insecticide‐treated net; LLIN: long‐lasting insecticidal net(s); PBO: piperonyl butoxide
Characteristics of studies awaiting assessment [ordered by study ID]
Koudou 2012.
| Methods | Village trial |
| Participants | Bouaké ‐ 100% An gambiae: (70% S‐form, 30% M‐form) Tiassalé ‐ 100% An gambiae: (70% S‐form, 30% M‐form) |
| Interventions | Control: LLIN, PermaNet 2.0 Extra, Intervention: LLIN, PermaNet 3.0 |
| Outcomes | Blood feeding, mosquito density |
| Mosquito Resistance Status | Bouaké ‐ resistant ‐ moderate (43.9% mortality, N = 114) Tiassalé ‐ resistant ‐ moderate (7.5% mortality, N = 106) |
| Net Treatment | Nets unholed and unwashed |
| Location(s) | Bouaké, Côte d’Ivoire Tiassalé, Côte d’Ivoire |
| Notes | Trial conducted: November 2009‐January 2012 |
Martine 2017.
| Methods | Cluster‐randomized controlled village trial |
| Participants | Wild mosquitoes in Muleba, Tanzania (An gambiae and An funestus) |
| Interventions | Control: LLIN, Olyset Net Intervention: LLIN, Olyset Plus |
| Outcomes | Physical integrity of the net Number of mosquitoes resting inside net Insecticidal activity |
| Mosquito Resistance Status | At baseline using adopted WHO resistance assays, field mosquitoes were 7%, 13% and 60%, 63% (An gambiae, An funestus) resistant to Olyset Plus and standard Olyset nets. |
| Net Treatment | Nets unholed and unwashed |
| Location(s) | Tanzania |
| Notes | Entomological trial that was part of Protopopoff 2018 |
N’Guessan 2016.
| Methods | Experimental hut trial |
| Participants | An gambiae |
| Interventions | Control: LLIN, MAGNet LN Intervention: LLIN, Veeralin LN |
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily |
| Mosquito Resistance Status | |
| Net Treatment | Nets holed, nets unwashed and washed (x 20) |
| Location(s) | M’be, Côte d’Ivoire |
| Notes |
Shono 2017.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Mosquito Resistance Status | Not available |
| Net Treatment | Control: LLIN, Olyset Net Intervention: LLIN, Olyset Plus |
| Location(s) | Not available |
| Notes |
Tungu 2017.
| Methods | Experimental hut trial |
| Participants | An funestus |
| Interventions | Control: LLIN, DawaPlus 2.0 Intervention: LLIN, DawaPlus 3.0, DawaPlus 4.0 |
| Outcomes | Mosquito mortality, blood feeding, deterrence, exophily |
| Mosquito Resistance Status | |
| Net Treatment | Nets holed, nets unwashed and washed (x 20) |
| Location(s) | Muheza, Tanzania |
| Notes |
Abbreviations: An funestus: Anopheles funestus;An gambiae: Anopheles gambiae; LLIN: long‐lasting insecticidal net(s)
Characteristics of ongoing studies [ordered by study ID]
ISRCTN17516395.
| Trial name or title | Impact of long‐lasting insecticide treated bed nets with and without piperonly butoxide (PBO) on malaria indictators in Uganda |
| Methods | Cluster‐randomized trial |
| Participants | Households with at least one adult resident and one child aged 2‐10 years. |
| Interventions | PermaNet 2.0 PermaNet 3.0 Olyset Net Olyset Plus |
| Outcomes | Primary outcomes; parasite prevalence (proportion of thick blood smears that are positive for asexual parasites) in children ages 2‐10 years, asssessed prior to net distribution, and up to 3 times after nets are distributed. Secondary outcomes; prevalence of anaemia and mean haemoglobin in children ages 2‐10 years, frequency of molecular markers associated with insecticide resistance in the primary malaria vector, prevalence of phenotypic insecticide resistance in mosquitoes. |
| Starting date | January 2017 |
| Contact information | Professor Martin Donnelly |
| Notes |
NCT03289663.
| Trial name or title | Effectiveness study of new generation bednets in the context of conventional insecticide resistance in the Democratic Republic of the Congo (Net‐PBO) |
| Methods | Cluster‐randomized trial |
| Participants | 1680 participants, 0‐10‐year‐old subjects in 30 villages |
| Interventions | Control: bed net treated with pyrethroid only Intervention: bed net treated with both pyrethroid and PBO |
| Outcomes | Incidence rate of laboratory confirmed clinical cases of Malaria (time frame: participants will be actively followed up for 12 months and any suspected case of clinical malaria will immediately lead to microscopy and RDT for confirmation.) Microscopy to confirm the diagnosis of malaria sporozoite rate (time frame: Anopheles mosquitoes will be captured every 3 months during 1 year), sporozoite detection by ELISA to determine infectivity of Anopheles |
| Starting date | 2 October 2017 |
| Contact information | |
| Notes |
Abbreviations: ELISA: enzyme‐linked immunosorbent assay; PBO: piperonyl butoxide
Differences between protocol and review
Previously PBO‐nets were classified as PBO‐LLINs, however as the durability of PBO on nets has not been classified as long lasting, these were subsequently referred to as pyrethroid‐PBO nets. As a result of this our review title changed from ‘Piperonyl butoxide (PBO) combined with pyrethroids in long‐lasting insecticidal nets (LLINs) to prevent malaria in Africa' to ‘Piperonyl butoxide (PBO) combined with pyrethroids in insecticide‐treated nets (ITNs) to prevent malaria in Africa'.
We added Leslie Choi as a review author.
Additional criteria for assessing the risk of bias of village trials were added. These are in line with the Cochrane ‘Risk of bias’ tool (Higgins 2017), and the five additional criteria listed in Section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions that relate specifically to cluster‐randomized trials (Higgins 2011).
The published protocol stated all stratified analysis factors under subgroup analysis (Gleave 2017). We have corrected this to state that subgroup analysis was only performed on whether nets were unwashed or washed.
Contributions of authors
KG, NL, and HR conceived and designed the protocol.
KG and NL conducted the trial screening, data extraction and analysis.
MR and LC provided statistical support.
KG, NL, and HR wrote the final manuscripts, and all review authors approved the final manuscript.
HR is the guarantor of the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Project number 300342‐104
-
World Health Organization (WHO), Switzerland.
WHO Global Malaria Programme Agreement for Performance of Work (APW) Grant 2017 (number 709319)
Declarations of interest
KG has no known conflicts of interest. NL has acted as rapporteur since 2015 for the Innovative Vector Control Consortium (IVCC) at their External Scientific Advisory Committee (ESAC) meetings. MR has no known conflicts of interest. LC has no known conflicts of interest. HR has served on a WHO committee to consider the evidence for PBO nets in malaria control. Preparation of the background work presented at this WHO meeting was funded by the Global Fund for AIDS, TB and Malaria. Although HR interacts regularly with bed net manufacturers through her own research and her role on the IVCC's advisory panels, neither HR nor her research group have received direct funding from these companies.
These authors contributed equally to this work.
These authors contributed equally to this work.
Unchanged
References
References to studies included in this review
Awolola 2014 {published data only}
- Awolola ST, Adeogun AO, Olojede JB, Oduola AO, Oyewole IO, Amajoh CN. Impact of PermaNet 3.0 on entomological indices in an area of pyrethroid resistant Anopheles gambiae in south‐western Nigeria. Parasites & Vectors 2014;7:236. [DOI: 10.1186/1756-3305-7-236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayili 2017 {published data only (unpublished sought but not used)}
- Bayili K. Phase II field evaluation of long‐lasting nets DawaPlus 3.0 (deltamethrin and PBO in roof panel; deltamethrin alone in the side panels) and DawaPlus 4.0 (deltamethrin and PBO) of Tana Netting against natural populations of Anopheles gambiae s.l in Burkina Faso. Report of the twentieth WHOPES working group meeting, WHO/HQ, Geneva, 20–24 March 2017. http://apps.who.int/iris/bitstream/handle/10665/258921/WHO‐HTM‐NTD‐WHOPES‐2017.04‐eng.pdf;jsessionid=87560DBD471E349CBB7A99796C513DBC?sequence=1 (accessed 13 September 2017). [WHO/HTM/NTD/WHOPES/2017.04]
Cisse 2017 {published and unpublished data}
- Cisse MB, Sangare D, Oxborough RM, Dicko A, Dengela D, Sadou A, et al. A village level cluster‐randomized entomological evaluation of combination long‐lasting insecticidal nets containing pyrethroid plus PBO synergist in Southern Mali. Malaria Journal 2017;16:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbel 2010 {published data only}
- Corbel V, Chabi J, Dabiré RK, Etang J, Nwane P, Pigeon O, et al. Field efficacy of a new mosaic long‐lasting mosquito net (PermaNet 3.0) against pyrethroid‐resistant malaria vectors: a multi centre study in Western and Central Africa. Malaria Journal 2010;9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darriet 2011 {published data only}
- Darriet F, Chandre F. Combining piperonyl butoxide and dinotefuran restores the efficacy of deltamethrin mosquito nets against resistant Anopheles gambiae (Diptera: Culicidae). Journal of Medical Entomology 2011;48(4):952‐5. [DOI] [PubMed] [Google Scholar]
Darriet 2013 {published data only}
- Darriet F, Chandre F. Efficacy of six neonicotinoid insecticides alone and in combination with deltamethrin and piperonyl butoxide against pyrethroid‐resistant Aedes aegypti and Anopheles gambiae (Diptera: Culicidae). Pest Management Science 2013;69(8):905‐10. [DOI] [PubMed] [Google Scholar]
Koudou 2011 {published data only}
- Koudou BG, Koffi AA, Malone D, Hemingway J. Efficacy of PermaNet® 2.0 and PermaNet® 3.0 against insecticide‐resistant Anopheles gambiae in experimental huts in Côte d’Ivoire. Malaria Journal 2011;10(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2016 {published and unpublished data}
- Moore S. Field evaluation of an alpha‐cypermethrin+PBO long‐lasting insecticidal net (Veeralin LN) against natural populations of Anopheles arabiensis in experimental huts, Tanzania [Unpublished report to the WHO Pesticide Evaluation Scheme (WHOPES)]. Report of the nineteenth WHOPES working group meeting: WHO/HQ, Geneva, 8–11 February 2016. http://apps.who.int/iris/bitstream/handle/10665/205588/9789241510400_eng.pdf?sequence=1 (accessed 24 August 2017). [WHO/HTM/NTD/WHOPES/2016.2]
Mzilahowa 2014 {unpublished data only}
- Mzilahowa T, Luka M, Chiumia M, Gimnig J. Efficacy of the PermaNet 3.0 and the Olyset Plus against pyrethroid resistant An funestus and An arabiensis (as supplied 14 March 2015). Data on file (received 14 March 2015).
N'Guessan 2010 {published data only}
- N’Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, Pigeon O, et al. An experimental hut evaluation of PermaNet(®) 3.0, a deltamethrin‐piperonyl butoxide combination net, against pyrethroid‐resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010;104(12):758‐65. [DOI] [PubMed] [Google Scholar]
Pennetier 2013 {published data only}
- Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, et al. Efficacy of Olyset(R) Plus, a new long‐lasting insecticidal net incorporating permethrin and piperonyl‐butoxide against multi‐resistant malaria vectors [corrected]. PLoS One 2013;8(10):e75134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Protopopoff 2018 {published data only}
- Protopopoff N, Charlwood D, Mosha J, Wright A, Kisinza W, Mosha F, et al. Evaluation of a novel long lasting insecticidal net to indoor residual spray product, separately to together, against malaria transmitted by pyrethroid resistant mosquitoes in northwest Tanzania: a cluster randomized controlled trial. Lancet 2018;391(10130):1577‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiles‐Ocran 2013 {unpublished data only}
- Stiles‐Ocran J. Field evaluation of PermaNet® 3.0 in controlling pyrethroid resistant Anopheles gambiae in the Chirano Area, Western Region, Ghana (as supplied 12 March 2015). Data on file 2013.
Toé 2018 {published and unpublished data}
- Toé KH, Müller P, Badolo A, Traore A, Sagnon N, Dabiré RK, et al. Do bednets including piperonyl butoxide offer additional protection against populations of Anopheles gambiae s.l. that are highly resistant to pyrethroids? An experimental hut evaluation in Burkina Faso. Medical and Veterinary Entomology 2018;32(4):407‐16. [DOI: 10.1111/mve.12316] [DOI] [PubMed] [Google Scholar]
Tungu 2010 {published data only}
- Tungu P, Magesa S, Maxwell C, Malima R, Masue D, Sudi W, et al. Evaluation of PermaNet 3.0 a deltamethrin‐PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malaria Journal 2010;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Koudou 2012 {unpublished data only}
- Koudou B, Malone D. Does PermaNet® 3.0 protect against pyrethroid resistant mosquitoes?. Manuscript in preparation for Acta Tropica (received 14 December 2012).
Martine 2017 {published data only}
- Martine JL, Protopopof N, Magesa S, Mosha FW. Effectiveness of two types of long lasting insecticidal nets after two years of use for malaria vector control in an area of high pyrethroid resistance, Muleba, Tanzania. Abstract 176. www.astmh.org/ASTMH/media/2017‐Annual‐Meeting/ASTMH‐2017‐Abstract‐Book.pdf (accessed 24 August 2017).
N’Guessan 2016 {published data only (unpublished sought but not used)}
- N’Guessan R. Experimental hut evaluation of an alphacypermethrin+ PBO mixture long‐lasting insecticidal net Veeralin LNof Vector Control Innovations Pvt Ltd., India against natural populations of Anopheles gambiae in M’Be, Côte d’Ivoire. Report of the nineteenth WHOPES working group meeting: WHO/HQ, Geneva, 8–11 February 2016. http://apps.who.int/iris/bitstream/handle/10665/205588/9789241510400_eng.pdf?sequence=1 (accessed 24 August 2017). [WHO/HTM/NTD/WHOPES/2016.2]
Shono 2017 {published data only}
- Shono Y, Ohashi K, Lucas JR. Biological performance of Olyset® Plus, a long‐lasting mosquito net incorporating a mixture of a pyrethroid and synergist. Acta Horticulturae 2017;1169:77‐81. [Google Scholar]
Tungu 2017 {published data only (unpublished sought but not used)}
- Tungu P. Phase II study to evaluate the efficacy and wash resistance of DawaPlus 3.0 and DawaPlus 4.0 against natural populations of Anopheles funestus s.s. in experimental huts in Muheza, Tanzania. Report of the twentieth WHOPES working group meeting, WHO/HQ, Geneva, 20–24 March 2017.. http://apps.who.int/iris/bitstream/handle/10665/258921/WHO‐HTM‐NTD‐WHOPES‐2017.04‐eng.pdf;jsessionid=87560DBD471E349CBB7A99796C513DBC?sequence=1 (accessed 13 September 2017). [WHO/HTM/NTD/WHOPES/2017.04]
References to ongoing studies
ISRCTN17516395 {unpublished data only}
- ISRCTN17516395. Impact of long‐lasting insecticide treated bednets with and without piperonyl butoxide (PBO) on malaria indicators in Uganda.. www.isrctn.com/ISRCTN17516395 (accessed 24 August 2018).
NCT03289663 {unpublished data only}
- NCT03289663. Effectiveness study of new generation bednets in the context of conventional insecticide resistance in the Democratic Republic of Congo. ClinicalTrials.gov/show/NCT03289663 (first received 24 August 2018).
Additional references
Abílio 2015
- Abílio AP, Marrune P, Deus N, Mbofana F, Muianga P, Kampango A. Bio‐efficacy of new long‐lasting insecticide‐treated bed nets against Anopheles funestus and Anopheles gambiae from central and northern Mozambique. Malaria Journal 2015;14:352. [DOI: 10.1186/s12936-015-0885-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adeogun 2012
- Adeogun AO, Olojede JB, Oduola AO, Awolola TS. Efficacy of a combination long lasting insecticidal net (PermaNet® 3.0) against pyrethroid resistant Anopheles gambiae s.s. and Culex quinquefasciatus: an experimental hut trial in Nigeria. Nigerian Journal of Clinical & Biomedical Research 2012;6:37‐50. [Google Scholar]
Aïzoun 2013
- Aïzoun N, Ossè R, Azondekon R, Alia R, Oussou O, Gnanguenon V, et al. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, West Africa. Parasites & Vectors 2013;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatt 2015
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015;526(7572):207‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bobanga 2013
- Bobanga T, Ayieko W, Zanga M, Umesumbu S, Landela A, Fataki O, et al. Field efficacy and acceptability of PermaNet® 3.0 and OlysetNet® in Kinshasa, Democratic Republic of the Congo. Journal of Vector Borne Diseases 2013;50(3):206‐14. [PubMed] [Google Scholar]
Churcher 2016
- Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 2016;5:e16090. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Djègbè 2011
- Djègbè I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malaria Journal 2011;10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 5 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hawley 2003
- Hawley WA, Phillips‐Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, et al. Community‐wide effects of permethrin‐treated bed nets on child mortality and malaria morbidity in western Kenya. American Journal of Tropical Medicine & Hygiene 2003;68(4 Suppl):121‐7. [DOI: 10.4269/ajtmh.2003.68.121] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Killeen 2018
- Killeen G, Ranson H. Insecticide‐resistant malaria vectors must be tackled. Lancet 2018;391(10130):1551‐2. [DOI] [PubMed] [Google Scholar]
Kleinschmidt 2018
- Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long‐lasting insecticidal nets: a WHO‐coordinated, prospective, international, observational cohort study. Lancet 2018;18:20172‐5. [DOI: 10.1016/S1473-3099(18)30172-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lengeler 2004
- Lengeler C. Insecticide‐treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000363.pub2] [DOI] [PubMed] [Google Scholar]
Maxwell 2002
- Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community‐wide use of insecticide‐treated nets for 3‐4 years on malarial morbidity in Tanzania. Tropical Medicine & International Health 2002;7(12):1003‐8. [DOI: 10.1046/j.1365-3156.2002.00966] [DOI] [PubMed] [Google Scholar]
Mitchell 2012
- Mitchell SN, Stevenson BJ, Müller P, Wilding CS, Egyir‐Yawson A, Field SG, et al. Identification and validation of a gene causing cross‐resistance between insecticide classes in Anopheles gambiae from Ghana. Proceedings of the Nationall Academy of Sciences of the United States of America 2012;109(16):6147‐52. [DOI: 10.1073/pnas.1203452109] [DOI] [PMC free article] [PubMed] [Google Scholar]
MPAC 2016
- Malaria Policy Advisory Committee. WHO Malaria Policy Advisory Committee (MPAC) meeting report (September 2016). www.who.int/malaria/publications/atoz/mpac‐september2016‐report.pdf?ua=1 (accessed 1 May 2017).
N'Guessan 2007
- N'Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide‐treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases 2007;13(2):199‐206. [DOI: 10.3201/eid1302.060631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2015
- Parker J, Angarita‐Jaimes N, Abe M, Towers CE, Towers D, McCall P. Infrared video tracking of Anopheles gambiae at insecticide‐treated bed nets reveals rapid decisive impact after brief localised net contact. Scientific Reports 2015;5:e13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
PMI 2018
- PMI. U.S. President’s Malaria Initiative technical guidance. www.pmi.gov/docs/default‐source/default‐document‐library/tools‐curricula/pmi‐technical‐guidance‐(february‐2017).pdf?sfvrsn=16 2018.
Ranson 2011
- Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African Anopheline mosquitoes: what are the implications for malaria control?. Trends in Parasitology 2011;27(2):91‐8. [DOI: 10.1016/j.pt.2010.08.004] [DOI] [PubMed] [Google Scholar]
Ranson 2016
- Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends in Parasitology 2016;32(3):187‐96. [DOI: 10.1016/j.pt.2015.11.010] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridl 2008
- Ridl FC, Bass C, Torrez M, Govender D, Ramdeen V, Yellot L, et al. A pre‐intervention study of malaria vector abundance in Rio Muni, Equatorial Guinea: their role in malaria transmission and the incidence of insecticide resistance alleles. Malaria Journal 2008;7:194. [DOI: 10.1186/1475-2875-7-194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riveron 2015
- Riveron JM, Chiumia M, Menze BD, Barnes KG, Irving H, Ibrahim SS, et al. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: a major concern for malaria vector control. Malaria Journal 2015;14:344. [DOI: 10.1186/s12936-015-0877-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stevenson 2011
- Stevenson BJ, Bibby J, Pignatelli P, Muangnoicharoen S, O'Neill PM, Lian LY, et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochemistry and Molecular Biology 2011;41(7):492‐502. [DOI: 10.1016/j.ibmb.2011.02.003] [DOI] [PubMed] [Google Scholar]
Strode 2014
- Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide‐treated bed nets against African Anopheline mosquitoes: systematic review and meta‐analysis. PLoS Medicine 2014;11(3):e1001619. [DOI: 10.1371/journal.pmed.1001619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sumitomo 2013
- Sumitomo Chemical Co. Ltd. Olyset Plus Technical Brochure 2013. sumivector.com/sites/default/files/site‐content/pdf/Olyset_Plus_Technical_Brochure_Jan_2013_ENG.pdf (accessed 1 May 2017).
Vestergaard 2015
- Vestergaard. Technical basis for deployment of PermaNet® 3.0 in areas with pyrethroid‐resistant malaria vectors. PermaNet® 3.0 Technical Brochure. January 2015. www.vestergaard.com/images/pdf/PN3_Tech_Eng_2015.pdf (accessed 1 May 2017).
WHO 2013
- World Health Organization Pesticide Evaluation Scheme. Guidelines for laboratory and field testing of long‐lasting insecticidal nets. apps.who.int/iris/bitstream/10665/80270/1/9789241505277_eng.pdf (accessed 24 August 2018).
WHO 2016
- World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Second Edition. Geneva: WHO, 2016. [Google Scholar]
WHO 2017a
- World Health Organization. World Malaria Report 2017. www.who.int/malaria/publications/world‐malaria‐report‐2017/en/ (accessed 24 August 2018).
WHO 2017b
- World Health Organization/Department of Control of Neglected Tropical Diseases. Design of epidemiological trials for vector control products, Report of a WHO Expert Advisory Group; Château de Penthes, Geneva, 24–25 April 2017. www.who.int/neglected_diseases/vector_ecology/resources/WHO_HTM_NTD_VEM_2017.04/en/ (accessed 11 July 2017).
WHO 2018
- World Health Organization. Transfer of product from Tana Netting FZ‐LLC to NRS Moon Netting FZE. www.who.int/pq‐vector‐control/prequalified‐lists/Transfer028‐001PQT‐VC20180911.pdf (accessed 4 November 2018).
WHO‐GMP 2015
- World Health Organization Global Malaria Programme. Conditions for use of long‐lasting insecticidal nets treated with a pyrethroid and piperonyl butoxide. www.who.int/malaria/areas/vector_control/use‐of‐pbo‐treated‐llins‐report‐nov2015.pdf. World Health Organization, (accessed 24 August 2018).
WHO‐GMP 2017a
- World Health Organization. Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide. apps.who.int/iris/bitstream/handle/10665/258939/WHO‐HTM‐GMP‐2017.17‐eng.pdf?sequence=1 (accessed 24 August 2018).
WHO‐GMP 2017b
- World Health Organization. The evaluation process for vector control products. www.who.int/malaria/publications/atoz/evaluation‐process‐vector‐control‐products/en/ (accessed 24 August 2018).
WHO‐GMP 2017c
- World Health Organization Global Malaria Programme. Malaria vector control policy recommendations and their applicability to product evaluation. www.who.int/malaria/publications/atoz/vector‐control‐recommendations/en/. World Health Organization, (accessed 24 August 2018).
WHOPES 2016
- WHO/Department of control of neglected tropical diseases. Report of the nineteenth WHOPES working group meeting: WHO/HQ, Geneva, 8–11 February 2016. Review of Veeralin LN, VectoMax GR, Bactivec SC. apps.who.int/iris/bitstream/10665/205588/1/9789241510400_eng.pdf (accessed 1 May 2017).
Yewhalaw 2012
- Yewhalaw D, Asale A, Tushune K, Getachew Y, Duchateau L, Speybroeck N. Bio‐efficacy of selected long‐lasting insecticidal nets against pyrethroid resistant Anopheles arabiensis from South‐Western Ethiopia. Parasites & Vectors 2012;5:159. [DOI: 10.1186/1756-3305-5-159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaim 2000
- Zaim M, Aitio A, Nakashima N. Safety of pyrethroid‐treated mosquito nets. Medical and Veterinary Entomology 2000;14(1):1‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gleave 2017
- Gleave K, Lissenden N, Richardson M, Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in long‐lasting insecticidal nets (LLINs) to prevent malaria in Africa. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012776] [DOI] [Google Scholar]


