Keywords: nerve regeneration, TRPA1, TRPV1, formaldehyde, menthol, CGRP, dorsal root ganglion, neuron, neurogenic inflammation, nociceptive signal, ERK1/2, neural regeneration
Abstract
Transient receptor potential ankyrin 1 (TRPA1) is a key player in pain and neurogenic inflammation, and is localized in nociceptive primary sensory dorsal root ganglion (DRG) neurons. TRPA1 plays a major role in the transmission of nociceptive sensory signals. The generation of neurogenic inflammation appears to involve TRPA1-evoked release of calcitonin gene-related peptide (CGRP). However, it remains unknown whether TRPA1 or CGRP expression is affected by TRPA1 activation. Thus, in this study, we examined TRPA1 and CGRP expression in DRG neurons in vitro after treatment with the TRPA1 activator formaldehyde or the TRPA1 blocker menthol. In addition, we examined the role of extracellular signal-regulated protein kinase 1/2 (ERK1/2) in this process. DRG neurons in culture were exposed to formaldehyde, menthol, the ERK1/2 inhibitor PD98059 + formaldehyde, or PD98059 + menthol. After treatment, real-time polymerase chain reaction, western blot assay and double immunofluorescence labeling were performed to evaluate TRPA1 and CGRP expression in DRG neurons. Formaldehyde elevated mRNA and protein levels of TRPA1 and CGRP, as well as the proportion of TRPA1- and CGRP-positive neurons. In contrast, menthol reduced TRPA1 and CGRP expression. Furthermore, the effects of formaldehyde, but not menthol, on CGRP expression were blocked by pretreatment with PD98059. PD98059 pretreatment did not affect TRPA1 expression in the presence of formaldehyde or menthol.
Chinese Library Classification No. R456; R338; R745
Introduction
Transient receptor potential ankyrin 1 (TRPA1) plays a key role in pain and neurogenic inflammation, according to its localization in nociceptive primary sensory dorsal root ganglion (DRG) neurons and its function as a non-selective cation channel (Leamy et al., 2011; Bertin et al., 2017; Masuoka et al., 2017). TRPA1 is closely associated with another major pain and neurogenic inflammation player, transient receptor potential vanilloid 1 (TRPV1), in terms of both expression and function (Anand et al., 2008; Iwasaki et al., 2008; Raisinghani et al., 2011). These two channels are expressed in skin-innervating sensory neurons, and are specifically activated by a wide range of environmental chemicals and temperatures that range from high burning heat to noxious cold (Strassmaier and Bakthavatchalam, 2011; Wang et al., 2012; Alpizar et al., 2013; Denner et al., 2017; Saito and Tominaga, 2017; Schwarz et al., 2017). As nocisensors, TRPA1 and TRPV1 mediate efferent signals via the secretion of neuropeptides, neurotransmitters and inflammatory signaling molecules. They also convey afferent signals from peripheral sensory nerve terminals via primary sensory nerve fibers to specific sites in the central nervous system (Benemei et al., 2017). Sensitization of TRPA1 and TRPV1 increases neuronal activity and contributes to hypersensitivity (Honda et al., 2017; Jardín et al., 2017; Negri and Maftei, 2017).
The involvement of TRPA1 in pain and inflammation and its localization in sensory neurons has been extensively studied (Bodkin and Brain, 2011; Ückert et al., 2017). The upregulation of TRPA1 by nerve growth factor could underlie in part the hyperalgesia induced by chronic inflammation (Diogenes et al., 2007; Luo et al., 2007). Nociception is associated with the activation of TRPA1, which induces extracellular signal-regulated protein kinase 1/2 (ERK1/2) phosphorylation in primary sensory DRG neurons (Donnerer et al., 2012). Accumulating evidence suggests that TRPA1 may be a promising drug target for treating pain (Zygmunt et al., 2014). Despite the increasing interest in TRPA1 as a therapeutic target, TRPA1 expression and activity in distinct subsets of DRG neurons remain unclear. During sensory signal processing, there is an intricate link between TRPA1, a nocisensor involved in inflammation, and calcitonin gene-related peptide (CGRP), a neurotransmitter involved in sensory signal transmission (Gajda et al., 2005; Schaeffer et al., 2010; Pozsgai et al., 2012). CGRP release from primary afferent neurons is stimulated by TRPA1 agonists. The increase of CGRP release is prevented by selective TRPA1 inhibition (Fischer et al., 2010; Kunkler et al., 2011). TRPA1 also participates in pain evoked by capsaicin-sensitive somatosensory neurons (Choi et al., 2011). Recently, it was shown that formaldehyde activates TRPA1 (McNamara et al., 2007; Sawynok and Reid, 2011). Interestingly, menthol inhibits TRPA1 (Macpherson et al, 2006). The biological activity of menthol was studied in cell culture and animal models because of its antipruritic and analgesic effects (Kamatou et al., 2013). The unique role of TRPA1 in mediating nociception has been recognized (Raisinghani et al., 2011). Therefore, TRPA1 and CGRP are potential novel therapeutic targets for relieving pain (Benemei et al, 2017; Berta et al, 2017; Demartini et al, 2017). Indeed, the differential expression of TRP cation channels contributes to the functional heterogeneity of nociception (Hjerling-Leffler et al., 2007). Understanding the mechanisms involved in regulating TRPA1 and CGRP expression in primary sensory neurons is of particular importance for elucidating the functions of TRPA1 and CGRP in nociceptive processing.
The activation of TRPA1 may impact the expression of TRPA1 or CGRP in primary sensory neurons. In the present study, we examine the effects of the TRPA1 agonist formaldehyde and the TRPA1 antagonist menthol on TRPA1 and CGRP expression in cultured primary DRG sensory neurons. We also investigate whether the ERK1/2 signaling pathway is involved in the modulation of TRPA1 and CGRP expression.
Materials and Methods
DRG cell culture
A total of 120 newborn rats (Wistar strain, < 24 hours after birth, 6–7 g in body weight, either sex) were used in this experiment. All animals (newborn rats) for this study were obtained from Shandong University, China (animal license No. SCXK (Lu) 20130009).
The animal protocols were approved by the Experimental Animal Ethics Committee of Shandong University, China (ethics approval No. 201402260001). During the experiments, all rats were anesthetized to minimize suffering. DRGs were removed, digested (0.25% trypsin; Sigma-Aldrich, St. Louis, MO, USA), centrifuged at 135 × g for 5 minutes, triturated, and seeded at 2 × 105 cells/well in 24-well plates (Costar, Corning, NY, USA). A coverslip covered with poly-L-lysine (Sigma) at 0.1 mg/mL concentration was placed in each well. D-MEM/F-12, supplemented with fetal bovine serum (5%), B-27 (2%) and L-glutamine (0.1 mg/mL), was used for cell culture in a humidified chamber at 37°C and 5% CO2. Then, 24 hours later, cytosine arabinoside (5 μg/mL) was added to remove non-neuronal cells. Subsequently, 24 hours later, cells were allowed to acclimate to the culture conditions for another 24 hours before observation.
Drug administration
After allowing the neurites to freely extend for 48 hours, the DRG neurons were pretreated with capsazepine (a TRPV1 antagonist, 10 μM) (Sigma-Aldrich) for 30 minutes. Thereafter, the neurons were treated and cultured for 24 hours as follows: (1) formaldehyde group (n = 5), DRG neurons were exposed to formaldehyde (Sigma-Aldrich) 10 μM; (2) menthol group (n = 5), DRG neurons were exposed to menthol (Sigma-Aldrich) 300 μM; (3) PD98059 + formaldehyde group (n = 5), PD98059 (an ERK1/2 inhibitor, 10 μM) (Cell Signaling Technology, Danvers, MA, USA) was added 30 minutes before formaldehyde (10 μM) exposure; (4) PD98059 + menthol group (n = 5), PD98059 (10 μM) was added 30 minutes prior to menthol (300 μM) exposure; (5) control group (n = 5), DRG neurons treated only with the TRPV1 receptor antagonist capsazepine (10 μM). Because TRPA1 and TRPV1 channels interact in neurons (Ruparel et al., 2011), capsazepine, a TRPV1 antagonist, was used to block the effects of TRPV1.
Real-time polymerase chain reaction (RT-PCR)
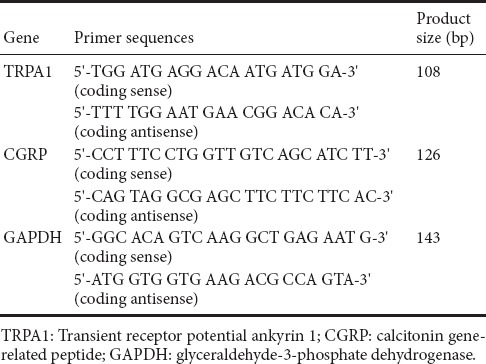
DRG neurons were cultured for 48 hours, and then treated with the different compounds for an additional 24 hours. Thereafter, TRPA1 and CGRP mRNAs, with GAPDH mRNA as an internal control, were detected by RT-PCR. Briefly, total RNA was isolated with TRIzol (TakaRa Biotechnology, Dalian, China). cDNA was synthesized using a cDNA synthesis kit (Thermo Scientific Molecular Biology, Vilnius, Lithuania). RT-PCR was performed using SYBR Green dye (Thermo Scientific Molecular Biology) according to the manufacturer’s instructions. The thermocycling parameters were 50°C for 2 minutes, 94°C for 15 minutes, followed by 40 cycles at 94°C for 15 seconds, 58°C for 30 seconds and 72°C for 30 seconds. The sequences of the oligonucleotide primers for TRPA1, CGRP and GAPDH are shown in Table 1. Relative quantification of the target gene was calculated with the 2–ΔΔCt method (Pfaffl, 2001).
Table 1.
Sequences of the oligonucleotide primers
Western blot assay
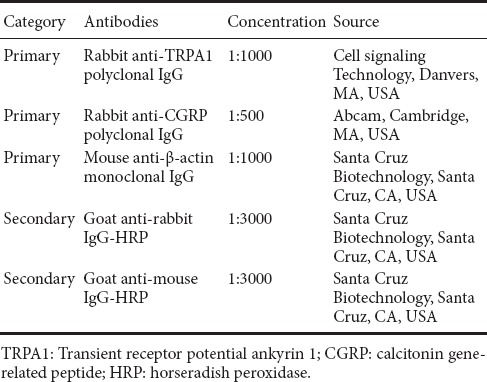
DRG neurons were cultured for 48 hours, and then treated with the different agents for an additional 24 hours. Subsequently, western blot assay was used to assess TRPA1 and CGRP protein expression levels. The procedure for the western blot assay was similar to that in a previous study (Bai et al., 2017). Briefly, RIPA lysis buffer with protease inhibitors (Amresco, Solon, OH, USA) was used for homogenizing cells. The samples were centrifuged at 10,000 ×g for 20 minutes, and the supernatants were collected. After determining the protein concentrations of the supernatants using the bicinchoninic acid assay, 50 μg of total protein for each sample was resolved by SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat milk for 2 hours at room temperature, and then incubated with the primary and secondary antibodies shown in Table 2. The immunoreactive bands were visualized with an enhanced chemiluminescence western blotting detection kit (Millipore, Billerica, MA, USA) using FluorChem E System (ProteinSimple, Santa Clara, CA, USA). The images were analyzed with ImageJ software (NIH Image, Bethesda, MD, USA). The protein levels of TRPA1 and CGRP were normalized to β-actin.
Table 2.
Primary and secondary antibodies for western blot assay
Immunofluorescence labeling
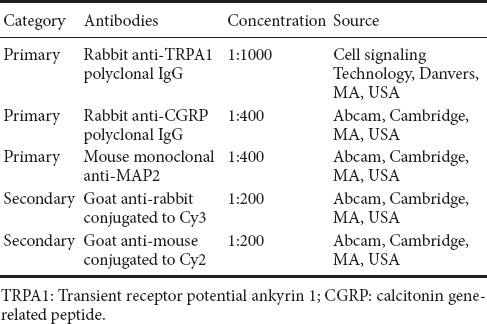
DRG neurons were cultured for 48 hours, and then treated with the different agents for an additional 24 hours. After that, microtubule-associated protein 2 (MAP2) antibody was used to label neurons. TRPA1 and CGRP antibodies were used for labeling the distinct neuronal subpopulations. Double fluorescent labeling was performed with MAP2 antibody + TRPA1 or CGRP antibody. The double fluorescent labeling procedure is described in our previous study (Li et al., 2016). Briefly, the cells were fixed in 4% paraformaldehyde for 40 minutes at 4°C, incubated with 0.6% Triton X-100 in 0.1 M PBS for 60 minutes at room temperature to permeabilize cells, and blocked with 10% normal goat serum for 120 minutes at room temperature. Subsequently, the cells were incubated with the primary and secondary antibodies shown in Table 3. The number of TRPA1-positive or CGRP-positive neurons was counted in five visual fields (one central visual field, plus the upper, lower, left and right visual fields) per coverslip using a fluorescence microscope (Olympus, Tokyo, Japan). The number of MAP2-positive neurons, which represent the total number of DRG neurons, was also counted in the same five visual fields. Then, the percentage of TRPA1-positive neurons or CGRP-positive neurons was calculated.
Table 3.
Primary and secondary antibodies for fluorescent labeling
Statistical analysis
SPSS 19.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. All quantitative data were expressed as the mean ± SD, and analyzed by one-way analysis of variance. Student-Newman-Keuls or Dunnett’s T3 post hoc test was used for analysis of the percentage of TRPA1-positive and CGRP-positive neurons. Dunnett’s T3 test was used to analyze TRPA1 and CGRP mRNA and protein levels. P < 0.05 was considered statistically significant.
Results
TRPA1 and CGRP mRNA levels
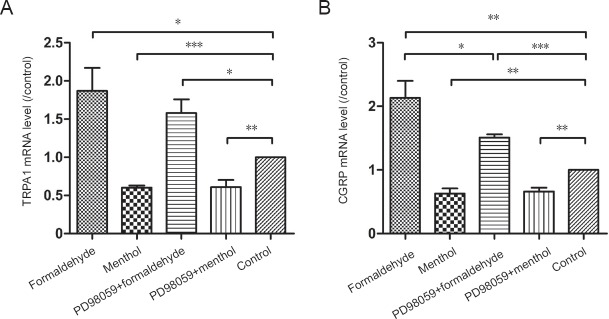
The changes in TRPA1 and CGRP mRNA expression after TRPA1 receptor activation or inhibition were assessed by RT-PCR analysis and one-way analysis of variance in the formaldehyde, menthol, PD98059 + formaldehyde, and PD98059 + menthol-treated cultures. Formaldehyde raised TRPA1 mRNA (P < 0.05) and CGRP mRNA (P < 0.01) levels, whereas menthol reduced TRPA1 mRNA (P < 0.001) and CGRP mRNA (P < 0.01) levels. Pretreatment with the ERK1/2 inhibitor PD98059 did not affect formaldehyde- or menthol-induced TRPA1 mRNA alterations. CGRP mRNA changes caused by formaldehyde (P < 0.05), but not menthol, were prevented by PD98059 pretreatment (Figure 1).
Figure 1.
Transient receptor potential ankyrin 1 (TRPA1) and calcitonin gene-related peptide (CGRP) mRNA levels in dorsal root ganglion (DRG) neurons.
(A) TRPA1 mRNA levels and (B) CGRP mRNA levels, expressed as a ratio to that in the control. Formaldehyde raised TRPA1 mRNA and CGRP mRNA levels. Menthol reduced TRPA1 mRNA and CGRP mRNA levels. The CGRP mRNA increase induced by formaldehyde was inhibited by PD98059. Data are expressed as the mean ± SD (n = 5, one-way analysis of variance followed by Dunnett’s T3 post hoc test). *P < 0.05, **P < 0.01, ***P < 0.001.
TRPA1 and CGRP protein levels
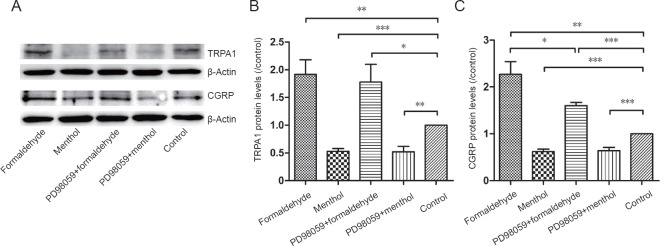
The changes in TRPA1 and CGRP protein expression produced by the various treatments were further evaluated by western blot assay and one-way analysis of variance in the formaldehyde, menthol, PD98059 + formaldehyde, and PD98059 + menthol-treated cultures. Formaldehyde increased TRPA1 (P < 0.01) and CGRP (P < 0.01) expression, while menthol inhibited TRPA1 (P < 0.001) and CGRP (P < 0.001) expression. Pretreatment with the ERK1/2 inhibitor PD98059 did not affect formaldehyde- or menthol-induced TRPA1 expression changes. CGRP expression changes caused by formaldehyde (P < 0.05), but not menthol, were blocked by PD98059 pretreatment (Figure 2).
Figure 2.
Protein levels of transient receptor potential ankyrin 1 (TRPA1) and calcitonin gene-related peptide (CGRP).
(A) Immunoblotting for TRPA1 and CGRP. (B, C) Optical density ratio of TRPA1 and CGRP protein relative to the control. Formaldehyde raised TRPA1 and CGRP protein levels. Menthol reduced TRPA1 and CGRP protein levels. The CGRP upregulation induced by formaldehyde was inhibited by PD98059. Data are expressed as the mean ± SD (n = 5, one-way analysis of variance followed by Dunnett’s T3 post hoc test). *P < 0.05, **P < 0.01, ***P < 0.001.
Proportion of TRPA1-positive neurons
TRPA1-positive neurons were detected with double fluorescent labeling for TRPA1 and MAP2. The changes in the percentage of TRPA1-positive neurons in the formaldehyde, menthol, PD98059 + formaldehyde, and PD98059 + menthol-treated cultures were analyzed by one-way analysis of variance. Compared with the control group, an increase in the proportion of TRPA1-positive neurons was observed after formaldehyde exposure (P < 0.001), and a decrease was observed after menthol exposure (P < 0.01). These results suggest that formaldehyde and menthol have differential effects on TRPA1 expression in distinct subpopulations of neurons. Inhibition of the ERK1/2 pathway with PD98059 did not affect formaldehyde-induced or menthol-induced TRPA1 expression changes (Figure 3).
Figure 3.
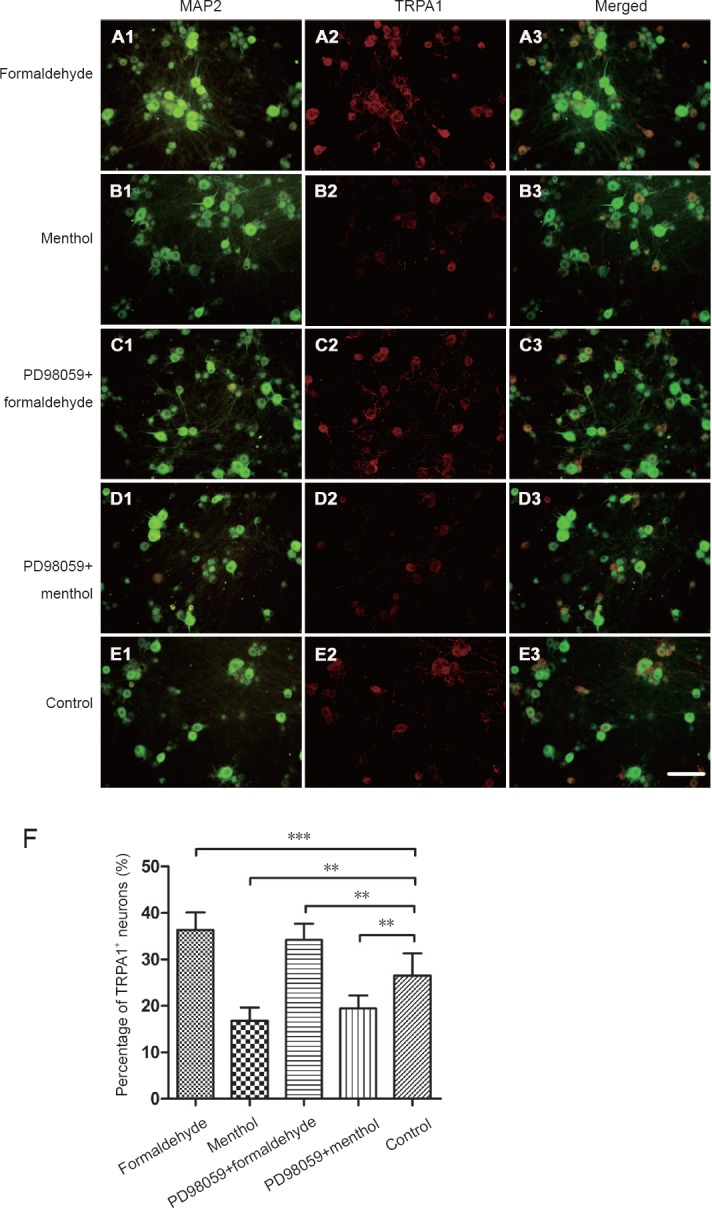
Double fluorescence for transient receptor potential ankyrin 1 (TRPA1) and microtubule-associated protein 2 (MAP2) in dorsal root ganglion (DRG) neurons.
TRPA1 was visualized with the Cy3-conjugated secondary antibody (red). MAP2 was visualized with the Cy2-conjugated secondary antibody (green). (A) Formaldehyde: DRG neurons were exposed to formaldehyde 10 μM. (B) Menthol: DRG neurons were exposed to menthol 300 μM. (C) PD98059 + formaldehyde: PD98059 (10 μM) was added 30 minutes before formaldehyde (10 μM) exposure. (D) PD98059 + menthol: PD98059 (10 μM) was added 30 minutes prior to menthol (300 μM) exposure. (E) Control group. Scale bar: 100 µm. (F) Histogram (mean ± SD) showing quantification of TRPA1-positive neurons. Formaldehyde elevated the proportion of TRPA1-positive neurons. Menthol reduced the proportion of TRPA1-positive neurons. The proportion of TRPA1-positive neurons is expressed as the mean ± SD (n = 5, one-way analysis of variance followed by Student-Newman-Keuls post hoc test). **P < 0.01, ***P < 0.001.
Proportion of CGRP-positive neurons
The number of CGRP-positive neurons may be modulated by peripheral stimulation, and changes in CGRP neurochemical phenotype may reflect the responsiveness of distinct CGRP neuronal subpopulations to peripheral nociceptor stimulation. Indeed, CGRP expression is restricted to a distinct subpopulation of DRGs (Kozłowska et al., 2017). The changes in the percentage of CGRP-positive neurons in formaldehyde, menthol, PD98059 + formaldehyde, and PD98059 + menthol-treated cultures were analyzed by one-way analysis of variance. Compared with the control group, the proportion of CGRP neurons was increased by formaldehyde exposure (P < 0.001), while it was decreased by menthol (P < 0.001). PD98059 prevented the change caused by formaldehyde (P < 0.01), but not that caused by menthol (Figure 4).
Figure 4.
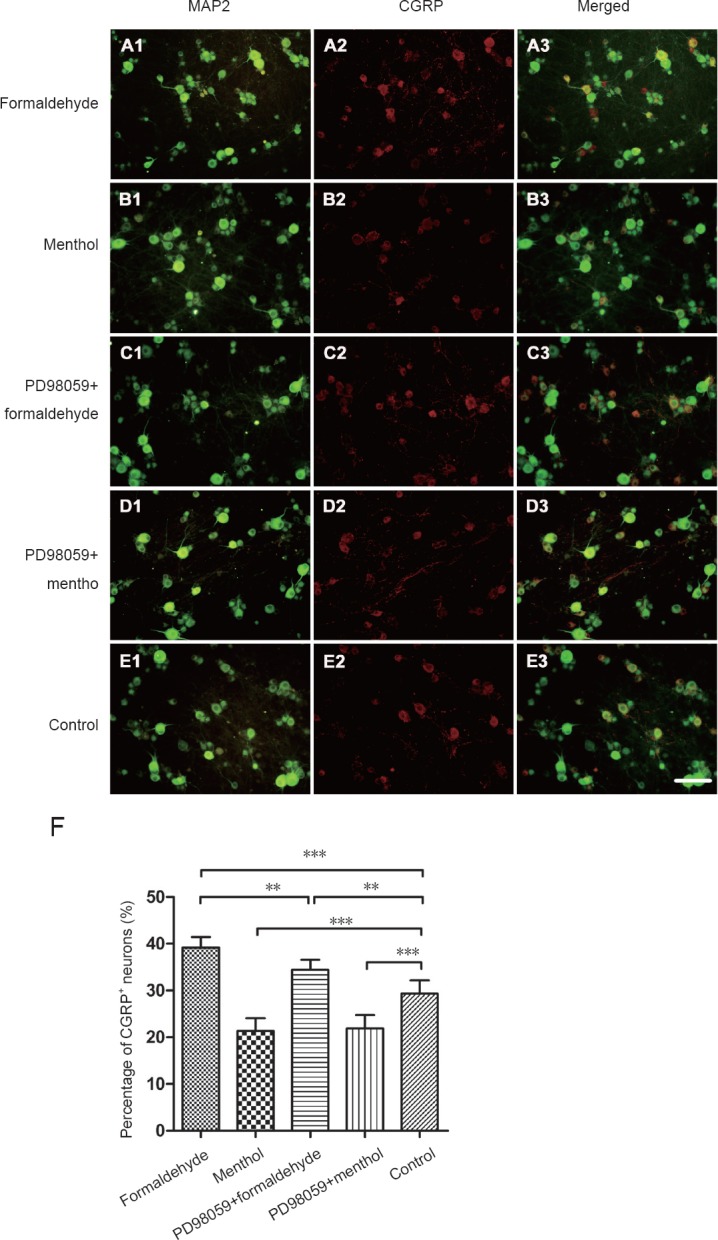
Double immunofluorescence for calcitonin gene-related peptide (CGRP) and microtubule-associated protein 2 (MAP2) in dorsal root ganglion (DRG) neurons.
CGRP was visualized with the Cy3-conjugated secondary antibody (red). MAP2 was visualized with the Cy2-conjugated secondary antibody (green). (A) Formaldehyde: DRG neurons were exposed to formaldehyde 10 μM. (B) Menthol: DRG neurons were exposed to menthol 300 μM. (C) PD98059 + formaldehyde: PD98059 (10 μM) was added 30 minutes before formaldehyde (10 μM) exposure. (D) PD98059 + menthol: PD98059 (10 μM) was added 30 minutes prior to menthol (300 μM) exposure. (E) Control group. The bar in the figure represents 100 µm. (F) Histogram (mean ± SD) for quantification of CGRP-positive neurons. Formaldehyde elevated the proportion of CGRP-positive neurons. Menthol reduced the proportion of CGRP-positive neurons. The increase in CGRP-positive neurons induced by formaldehyde was inhibited by PD98059. The proportion of CGRP-positive neurons is expressed as the mean ± SD (n = 5, one-way analysis of variance followed by Student-Newman-Keuls post hoc test). **P < 0.01, ***P < 0.001.
Discussion
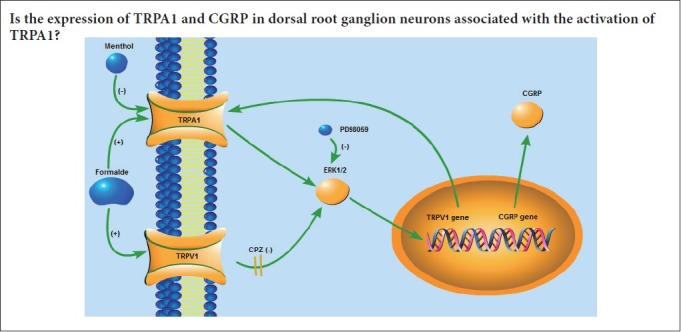
TRPA1, as a cellular sensor, plays a key role in transducing noxious stimuli, and in the generation of neurogenic inflammation and hyperalgesia (Kádková et al, 2017; Xie et al., 2018), perhaps through TRPA1 activation-induced CGRP release (Ushio et al., 2013; Borkum, 2018). A subset of TRPA1-expressing nociceptors produces and releases CGRP, which mediates neurogenic inflammatory responses (Nassini et al., 2014). Although TRPA1 is activated or inhibited by several compounds, the mechanisms underlying the TRPA1 and CGRP expression changes are unclear. In the present study, we investigated the effects of formaldehyde, a TRPA1 activator, and menthol, a TRPA1 blocker, on TRPA1 and CGRP expression in primary cultures of sensory neurons isolated from newborn rat DRGs. Formaldehyde and menthol not only play different roles in nociceptive signaling, as reported previously, but they also have differential effects on TPPA1 and CGRP expression, as observed in the present study. These effects of formaldehyde and menthol on TRPA1 and CGRP provide new insight into the mechanisms of nociception. Because formaldehyde may also activate TRPV1, a TRPV1 antagonist, capsazepine, was used in the present study.
Diabetes-related overexpression of TRPA1 may potentiate cold hyperalgesia in streptozotocin-induced diabetic rats (Barrière et al., 2012). Although formaldehyde activates TRPA1, and capsaicin activates TRPV1, DRG neurons from TRPA1-deficient mice (Bautista et al., 2006; Kwan et al., 2006) are insensitive to formaldehyde and retain the ability for capsaicin-induced TRPV1 activation (Kerstein et al., 2009). A previous study showed that formaldehyde activates TRPA1 in a distinct subpopulation of DRG neurons. This channel activation is caused by the covalent modification of cysteine within TRPA1, resulting in pain transmission by various signaling pathways (Macpherson et al., 2007).
The analgesic effect of menthol likely derives from its ability to inhibit TRPA1 (Macpherson et al., 2006). TRPA1 is frequently coexpressed with TRPV1 in neurons from the DRG and the trigeminal ganglion (Babes et al., 2004; Akopian et al., 2007; Salas et al., 2009; Staruschenko et al., 2010). Direct interactions between TRPA1 and TRPV1 in sensory neurons have been shown recently. Certain intrinsic characteristics of the TRPA1 channel are influenced by TRPV1. Furthermore, it has been demonstrated that the modulation of TRPV1 affects TRPA1 activity via several signaling pathways to influence desensitization (Akopian et al., 2007). The TRPA1 agonist formaldehyde also activates TRPV1 in neurons from the DRG (Tian et al., 2009). Desensitization of TRPV1 caused by TRPA1 inhibition leads to the suppression of TRPA1, which suggests that TRPA1 and TRPV1 directly interact (Ruparel et al., 2011). Because formaldehyde also activates TRPV1, TRPV1 activity was blocked by pretreatment with capsazepine to prevent TRPV1 from impacting TRPA1 activity. Therefore, the effects of formaldehyde and menthol on TRPA1 and CGRP expression observed in the present study are likely mediated by TRPA1, and not by TRPV1.
Our current results suggest that TRPA1 activation upregulates TRPA1 and CGRP expression, whereas TRPA1 inhibition has the converse effect. These findings imply that formaldehyde not only directly activates TRPA1 to induce CGRP release, but also indirectly by upregulating TRPA1 and CGRP expression. Similarly, menthol not only directly inhibits TRPA1 to block CGRP release, but also indirectly by downregulating TRPA1 and CGRP expression. Upregulation of TRPA1 and CGRP in distinct subpopulations of DRG neurons is associated with an increase in neuronal activity and hyperalgesia (Ikeda-Miyagawa et al., 2015), whereas a decrease in TRPA1 and CGRP is associated with a reduction in neuronal activity and anti-hyperalgesia. Accordingly, TRPA1 and its related neuropeptides very likely contribute to the regulation of neuropathic pain.
It has been suggested that TRPA1 has a major role in nociceptive processes, and that selective TRPA1 antagonists might possess anti-nociceptive properties (Andrade et al., 2008). In this study, the expression of both TRPA1 and CGRP was modulated by the TRPA1 agonist formaldehyde and by the TRPA1 antagonist menthol. These data suggest that persistent exposure to formaldehyde would promote TRPA1 and CGRP expression, whereas persistent exposure to menthol would inhibit TRPA1 and CGRP expression. Our findings are consistent with a recent study on incision-induced TRPA1 upregulation in trigeminal ganglion primary sensory neurons (Urata et al., 2015). Our findings are also consistent with another recent study showing that the TRPA1 antagonist camphor suppresses CGRP production (Mihara and Shibamoto, 2015). Therefore, the expression patterns of TRPA1 and CGRP should be taken into consideration when targeting TRPA1 as a potential anti-nociceptive therapeutic strategy.
The formaldehyde-induced nociceptive response is related to formaldehyde-specific TRP channels (Coste et al., 2012). TRPA1 activation leads to the phosphorylation of ERK1/2 in DRG neurons from rats (Donnerer et al, 2009; Donnerer and Liebmann, 2011; Donnerer et al., 2012; Kondo et al., 2013). In the present study, the changes in CGRP expression induced by formaldehyde were partially blocked by pretreatment with PD98059. This suggests that the ERK1/2 signaling pathway participates in the modulation of CGRP expression by formaldehyde. Interestingly, PD98059 did not affect the menthol-induced reduction in CGRP expression. It is possible that by blocking TRPA1 activation, menthol circumvents ERK1/2, which is downstream of TRPA1. Additionally, PD98059 did not impact the effects of formaldehyde and menthol on TRPA1 expression. This suggests that the ERK1/2 pathway does not modulate TRPA1 expression. Furthermore, the effect of PD98059 on the formaldehyde-induced change in CGRP expression was very weak, suggesting that ERK signaling does not substantially affect expression of the upstream TRPA1 receptor. Further studies by using organotypic DRG explant cultures, which might have mimicked in vivo conditions, should be carried out for testing different effects of formaldehyde and menthol. Furthermore, the noxious effect of formaldehyde and the analgesic effect of menthol should be further investigated with animal models following peripheral exposure.
In conclusion, the TRPA1 agonist formaldehyde increased TRPA1 and CGRP expression, whereas the TRPA1 antagonist menthol decreased their expression. The upregulation of CGRP caused by formaldehyde might be through activation of the ERK1/2 signaling pathway. Our novel findings provide a foundation for further study of the expression and function of TRPA1 and CGRP in nociceptive processing.
Footnotes
Conflicts of interest: The authors declare that there is no duality of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81501935 (to HL); the Natural Science Foundation of Shandong Province of China, No. ZR2014HQ065 (to HL). The funding sources had no role in the study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures were approved by the Experimental Animal Ethics Committee of Shandong University, China (ethical approval No. 201402260001). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81501935 (to HL); the Natural Science Foundation of Shandong Province of China, No. ZR2014HQ065 (to HL).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Norman C,Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpizar YA, Gees M, Sanchez A, Apetrei A, Voets T, Nilius B, Talavera K. Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Pflugers Arch. 2013;465:853–864. doi: 10.1007/s00424-012-1204-x. [DOI] [PubMed] [Google Scholar]
- 3.Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Andrade EL, Luiz AP, Ferreira J, Calixto JB. Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience. 2008;152:511–520. doi: 10.1016/j.neuroscience.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 6.Bai X, Chen T, Gao Y, Li H, Li Z, Liu Z. The protective effects of insulin-like growth factor-1 on neurochemical phenotypes of dorsal rootganglion neurons with BDE-209-induced neurotoxicity in vitro. Toxicol Ind Health. 2017;33:250–264. doi: 10.1177/0748233716638004. [DOI] [PubMed] [Google Scholar]
- 7.Barrière DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 2012;153:553–561. doi: 10.1016/j.pain.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Benemei S, De Logu F, Li Puma S, Marone IM, Coppi E, Ugolini F, Liedtke W, Pollastro F, Appendino G, Geppetti P, Materazzi S, Nassini R. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol. 2017;174:2897–2911. doi: 10.1111/bph.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21:695–703. doi: 10.1080/14728222.2017.1328057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertin S, Aoki-Nonaka Y, Lee J, de Jong PR, Kim P, Han T, Yu T, To K, Takahashi N, Boland BS, Chang JT, Ho SB, Herdman S, Corr M, Franco A, Sharma S, Dong H, Akopian AN, Raz E. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut. 2017;66:1584–1596. doi: 10.1136/gutjnl-2015-310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodkin JV, Brain SD. Transient receptor potential ankyrin 1: emerging pharmacology and indications for cardiovascular biology. Acta Physiol (Oxf) 2011;203:87–98. doi: 10.1111/j.1748-1716.2010.02203.x. [DOI] [PubMed] [Google Scholar]
- 13.Borkum JM. Harnessing migraines for neural regeneration. Neural Regen Res. 2018;13:609–615. doi: 10.4103/1673-5374.230275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi MJ, Jin Z, Park YS, Rhee YK, Jin YH. Transient receptor potential (TRP) A1 activated currents in TRPV1 and cholecystokinin-sensitive cranial visceral afferent neurons. Brain Res. 2011;1383:36–42. doi: 10.1016/j.brainres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Coste O, Möser CV, Sisignano M, Kynast KL, Minden A, Geisslinger G, Niederberger E. The p21-activated kinase PAK 5 is involved in formalin-induced nociception through regulation of MAP-kinase signaling and formalin-specific receptors. Behav Brain Res. 2012;234:121–128. doi: 10.1016/j.bbr.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Demartini C, Tassorelli C, Zanaboni AM, Tonsi G, Francesconi O, Nativi C, Greco R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: evaluation in an animal model. J Headache Pain. 2017;18:94. doi: 10.1186/s10194-017-0804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denner AC, Vogler B, Messlinger K, De Col R. Role of transient receptor potential ankyrin 1 receptors in rodent models of meningeal nociception - Experiments in vitro. Eur J Pain. 2017;21:843–854. doi: 10.1002/ejp.986. [DOI] [PubMed] [Google Scholar]
- 18.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain (2007) J Dent Res. 86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 19.Donnerer J, Liebmann I, Schuligoi R. Capsaicin- and mustard oil-induced extracellular signal-regulated protein kinase phosphorylation in sensory neurons in vivo: effects of neurokinins 1 and 2 receptor antagonists and of a nitric oxide synthase inhibitor. Basic Clin Pharmacol Toxicol. 2009;104:11–16. doi: 10.1111/j.1742-7843.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 20.Donnerer J, Liebmann I. A fluorescence-immunohistochemical study on phosphorylation of ERK1/2, p38, and STAT3 in rat dorsal root ganglia following noxious stimulation of hind paw sensory neurons. Tissue Cell. 2011;43:178–189. doi: 10.1016/j.tice.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Donnerer J, Liebmann I. Phosphorylation of ERK1/2 in dorsal root ganglia following sequential mustard oil and thermal stimulation of the rat hind paw. Pharmacology. 2012;89:7–12. doi: 10.1159/000334933. [DOI] [PubMed] [Google Scholar]
- 22.Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285:34781–34792. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gajda M, Litwin JA, Cichocki T, Timmermans JP, Adriaensen D. Development of sensory innervation in rat tibia: co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43) J Anat. 2005;207:135–144. doi: 10.1111/j.1469-7580.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda K, Shinoda M, Kondo M, Shimizu K, Yonemoto H, Otsuki K, Akasaka R, Furukawa A, Iwata K. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain. 2017;158:1754–1764. doi: 10.1097/j.pain.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda-Miyagawa Y, Kobayashi K, Yamanaka H, Okubo M, Wang S, Dai Y, Yagi H, Hirose M, Noguchi K. Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons. Mol Pain. 2015;11:8. doi: 10.1186/s12990-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki Y, Tanabe M, Kobata K, Watanabe T. TRPA1 agonists-allyl isothiocyanate and cinnamaldehyde-induce adrenaline secretion. Biosci Biotechnol Biochem. 2008;72:2608–2614. doi: 10.1271/bbb.80289. [DOI] [PubMed] [Google Scholar]
- 28.Jardín I, López JJ, Diez R, Sánchez-Collado J, Cantonero C, Albarrán L, Woodard GE, Redondo PC, Salido GM, Smani T, Rosado JA. TRPs in pain sensation. Front Physiol. 2017;8:392. doi: 10.3389/fphys.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kádková A, Synytsya V, Krusek J, Zímová L, Vlachová V. Molecular basis of TRPA1 regulation in nociceptive neurons. A review. Physiol Res. 2017;66:425–439. doi: 10.33549/physiolres.933553. [DOI] [PubMed] [Google Scholar]
- 30.Kamatou GP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. doi: 10.1016/j.phytochem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Kerstein PC, del Camino D, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo T, Sakurai J, Miwa H, Noguchi K. Activation of p38 MAPK through transient receptor potential A1 in a rat model of gastric distension-induced visceral pain. Neuroreport. 2013;24:68–72. doi: 10.1097/WNR.0b013e32835c7df2. [DOI] [PubMed] [Google Scholar]
- 33.Kozłowska A, Mikołajczyk A, Adamiak Z, Majewski M. Distribution and chemical coding of sensory neurons innervating the skin of the porcine hindlimb. Neuropeptides. 2017;61:1–14. doi: 10.1016/j.npep.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Leamy AW, Shukla P, McAlexander MA, Carr MJ, Ghatta S. Curcumin ((E, E)-1, 7-bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci Lett. 2011;503:157–162. doi: 10.1016/j.neulet.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Liu Z, Chi H, Bi Y, Song L, Liu H. The effects of IGF-1 on Trk expressing DRG neurons with HIV-gp120- induced neurotoxicity. Curr HIV Res. 2016;14:154–164. doi: 10.2174/1570162x14888151221163150. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuoka T, Kudo M, Yamashita Y, Yoshida J, Imaizumi N, Muramatsu I, Nishio M, Ishibashi T. TRPA1 channels modify TRPV1-mediated current responses in dorsal root ganglion neurons. Front Physiol. 2017;8:272. doi: 10.3389/fphys.2017.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihara S, Shibamoto T. The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin Immunol. 2015;11:11. doi: 10.1186/s13223-015-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 45.Negri L, Maftei D. Targeting the prokineticin system to control chronic pain and inflammation. Curr Med Chem. 2017 doi: 10.2174/0929867324666170713102514. doi: 10.2174/0929867324666170713102514. [DOI] [PubMed] [Google Scholar]
- 46.Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemény Á, Materazzi S, Nassini R, Helyes Z, Szolcsányi J, Pintér E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol. 2012;689:56–64. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 47.Raisinghani M, Zhong L, Jeffry JA, Bishnoi M, Pabbidi RM, Pimentel F, Cao DS, Evans MS, Premkumar LS. Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am J Physiol Cell Physiol. 2011;301:C587–C600. doi: 10.1152/ajpcell.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Mol Pharmacol. 2011;80:117–123. doi: 10.1124/mol.110.068940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito S, Tominaga M. Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates. Temperature (Austin) 2017;4:141–152. doi: 10.1080/23328940.2017.1315478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawynok J, Reid AR. Reduction of formalin-evoked responses and maintenance of peripheral antinociception by morphine against formalin in the spared nerve injury model. Neurosci Lett. 2011;494:99–103. doi: 10.1016/j.neulet.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 52.Schaeffer V, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Progress in dorsal root ganglion neurosteroidogenic activity: basic evidence and pathophysiological correlation. Prog Neurobiol. 2010;92:33–41. doi: 10.1016/j.pneurobio.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz MG, Namer B, Reeh PW, Fischer MJM. TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J Pain. 2017;18:526–534. doi: 10.1016/j.jpain.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem. 2010;285:15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strassmaier T, Bakthavatchalam R. Transient receptor potential A1 modulators. Curr Top Med Chem. 2011;11:2227–2236. doi: 10.2174/156802611796904915. [DOI] [PubMed] [Google Scholar]
- 56.Tian LJ, DU YR, Xiao Y, Lv ZM, Yu YQ, Cui XY, Chen J. Mediating roles of the vanilloid receptor TRPV1 in activation of rat primary afferent nociceptive neurons by formaldehyde. Sheng Li Xue Bao. 2009;61:404–416. [PubMed] [Google Scholar]
- 57.Ückert S, Albrecht K, Bannowsky A, Sohn M, Kuczyk MA, Hedlund P. Expression and distribution of the transient receptor potential cationic channel A1 (TRPA1) in the human clitoris-comparison to male penile erectile tissue. Int J Impot Res. 2017;29:179–183. doi: 10.1038/ijir.2017.15. [DOI] [PubMed] [Google Scholar]
- 58.Urata K, Shinoda M, Honda K, Lee J, Maruno M, Ito R, Gionhaku N, Iwata K. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J Dent Res. 2015;94:446–454. doi: 10.1177/0022034514565645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ushio N, Dai Y, Wang S, Fukuoka T, Noguchi K. Transient receptor potential channel A1 involved in calcitonin gene-related peptide release in neurons. Neural Regen Res. 2013;8:3013–3019. doi: 10.3969/j.issn.1673-5374.2013.32.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S, Lee J, Ro JY, Chung MK. Warmth suppresses and desensitizes damage-sensing ion channel TRPA1. Mol Pain. 2012;8:22. doi: 10.1186/1744-8069-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie HT, Xia ZY, Pan X, Zhao B, Liu ZG. Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury. Neural Regen Res. 2018;13:1263–1268. doi: 10.4103/1673-5374.235074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zygmunt PM, Högestätt ED. TRPA1. Handb Exp Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]