Abstract
OBJECTIVE:
The objective of this study is to summarize and analyze the brain signal patterns of empathy for pain caused by facial expressions of pain utilizing activation likelihood estimation, a meta-analysis method.
DATA SOURCES:
Studies concerning the brain mechanism were searched from the Science Citation Index, Science Direct, PubMed, DeepDyve, Cochrane Library, SinoMed, Wanfang, VIP, China National Knowledge Infrastructure, and other databases, such as SpringerLink, AMA, Science Online, Wiley Online, were collected. A time limitation of up to 13 December 2016 was applied to this study.
DATA SELECTION:
Studies presenting with all of the following criteria were considered for study inclusion: Use of functional magnetic resonance imaging, neutral and pained facial expression stimuli, involvement of adult healthy human participants over 18 years of age, whose empathy ability showed no difference from the healthy adult, a painless basic state, results presented in Talairach or Montreal Neurological Institute coordinates, multiple studies by the same team as long as they used different raw data.
OUTCOME MEASURES:
Activation likelihood estimation was used to calculate the combined main activated brain regions under the stimulation of pained facial expression.
RESULTS:
Eight studies were included, containing 178 subjects. Meta-analysis results suggested that the anterior cingulate cortex (BA32), anterior central gyrus (BA44), fusiform gyrus, and insula (BA13) were activated positively as major brain areas under the stimulation of pained facial expression.
CONCLUSION:
Our study shows that pained facial expression alone, without viewing of painful stimuli, activated brain regions related to pain empathy, further contributing to revealing the brain’s mechanisms of pain empathy.
Keywords: nerve regeneration, facial expression, pain empathy, functional magnetic resonance imaging, GringleALE, activation likelihood estimation, brain function imaging, anterior cingulate cortex, anterior central gyrus, fusiform gyrus, insula, neural regeneration
Chinese Library Classification No. R445; R741
Introduction
“Empathy” refers to the feeling and understanding of others’ emotions and thoughts. Pain, a relatively subjective experience, can elicit our empathy. Empathy for pain is a process in which people perceive, evaluate, and respond to the pain of others (Decety et al., 2008; Loggia et al., 2008).
Study of the brain’s mechanisms of pain is progressing because of the development of neurophysiology and the wider application of imaging technology (Davis et al., 1997) in recent years. Empathy induced by pained expressions has realistic significance. Healthcare workers can be alert to patients’ pain by watching their facial expressions. Picture-based paradigms (Simon et al., 2008) and cue-based paradigms (Lamm et al., 2011) are the main patterns applied to the study of empathy for pain.
Presenting a series of images evokes empathy for pain from subjects in the former paradigm. Most of the images depict painful injuries in limbs or neutral situations (Kam et al., 2014), and some record pained expressions (Vachon-Presseau et al., 2012). Abstract visuals are applied in the cue-based paradigm. Most studies suggest that the insular and cingulate cortices are activated by empathy (Singer and Lamm, 2009). Brain mechanisms differ because of diverse factors, including positive and negative experience (Enzi et al., 2016). The brain regions activated can change according to changes in activation intensity. However, there are few studies concerning the mechanisms of empathy for pain elicited by pained expressions.
Our aim was to perform a meta-analysis to integrate and interpret the relevant functional magnetic resonance imaging (fMRI) studies based on the differences in brain patterns elicited by pain. Using the activation likelihood estimation (ALE) method, the activated brain regions of merged samples were analyzed to explore the brain signal patterns associated with empathy for pain.
ALE is a kind of analysis method that is based on the voxel coordinates of the function of the brain regions positioning (Turkeltaub et al., 2002). ALE uses the three-dimensional Gaussian function smoothness and arranges inspection into the activated regional center coordinates described in multiple papers to define accurate positions of the activated brain regions.
Compared with traditional chart review, the greatest advantage of ALE lies in its objective and quantitative analysis; in other words, ALE works in a voxel-by-voxel fashion (Laird et al., 2009), to avoid the subjectivity of traditional chart review.
Data and Methods
Literature retrieval
Studies written in English were retrieved from Science Citation Index, Science Direct, PubMed, DeepDyve, Cochrane Library, SinoMed, Wanfang, VIP, China National Knowledge Infrastructure, and other databases, such as SpringerLink, American Medical Association, Science Online, and Wiley Online. The research reported herein was undertaken with a time limitation of up to 13 December 2016.
The key words were “facial AND expression AND pain AND empathy AND fMRI (or functional MRI)”. All authors participated in the literature search by computer and secondary search by hand. Unpublished literature was not included.
Inclusion and exclusion criteria
Inclusion criteria
The studies were randomized controlled trials; the blinding method and the allocation concealment scheme were not taken into account when developing inclusion and exclusion criteria. Studies presenting with all of the following criteria were considered for study inclusion:
-
(1)
Use of fMRI;
-
(2)
The subjects were shown neutral and pained facial expression stimuli;
-
(3)
The experiments involved adult healthy human participants. The basic state was painless.
-
(4)
The subjects were over 18 years of age.
-
(5)
The results were presented in Talairach or Montreal Neurological Institute coordinates.
-
(6)
Multiple studies by the same team as long as they used different raw data.
Exclusion criteria
Studies with one or more of the following conditions were excluded from this analysis:
-
(1)
Studies with data other than only facial expressions or short video stimulation.
-
(2)
In addition to facial expressions, other stimuli were displayed in the picture or video.
-
(3)
Animal studies.
-
(4)
Abstracts, summaries, case reports, minutes of meetings, lectures, and reviews.
-
(5)
Unpublished literature.
Interventions
The experimental group looked at a picture or short video, showing a volunteer with a pained expression. The control group looked at a picture or short video showing a volunteer with a neutral expression.
Literature screening and data extraction
The data collected included:
Title, author, and publication date.
Experimental group and (if used) control group information: Number of trials, proportion of male and female participants, average age, basic state, interventions, outcome indicators, and scanning methods.
Brain-related data: Brain area, hemisphere, voxel value (x, y, z), coordinate system, t value, and z value.
Statistical information: statistical methods, positive and negative activation.
Data processing and analysis
Coordinate transformation
The Lancaster (icbm2tal) transformation in GingerALE 2.3.6 software (Research Imaging Institute, San Antonio, TX, USA) was used to convert all coordinates to the Talairach coordinate system, and then all the converted coordinates were input into GingerALE 2.3.6 software for processing. A single data analysis was performed.
Parameter setting
According to the maximum activation coordinates peak and the three-dimensional Gaussian distribution model, the specific parameters were set as follows: P value ≤ 0.01, minimum cluster volume > 200 mm3. There was sufficient data to calculate brain ALE distribution.
Image processing
Mango 4.0.2 software (Research Imaging Institute) was used to map the three-dimensional coordinate data to the brain anatomical background of the Talairach coordinate space.
Results
Retrieval results
A total of 440 studies were retrieved. Based on the title and abstract, 392 studies were excluded, including abstracts, summaries, minutes of meetings, lectures, and reviews, as well as non-fMRI studies. The full text of the remaining 48 studies was read by the two authors. Applying the inclusion criteria, exclusion criteria, and interventions, 36 studies were initially considered and excluded. The full texts of the remaining 12 studies were read for the second time, and 4 studies with repetitive data or of low quality were excluded, leaving eight studies. Eight studies were considered in the final analysis (Budell et al., 1932; Botvinick et al., 2005; Simon et al., 2007; Danziger et al., 2009; Budell and Jackson, 2010; Eickhoff et al., 2012; Sheng et al., 2013; Hadjikhani et al., 2014) (Figure 1).
Figure 1.
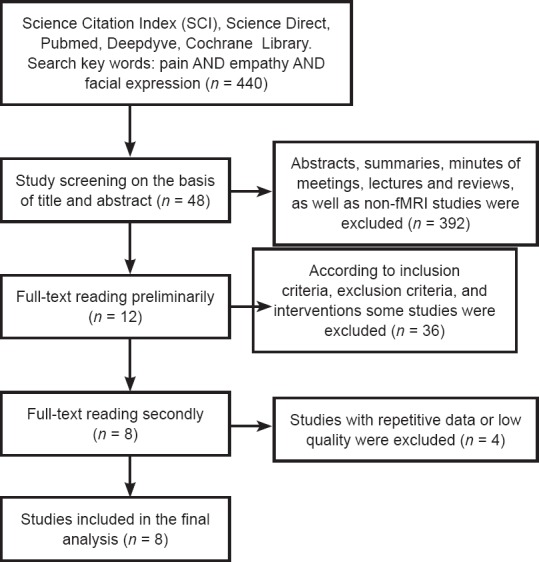
Flow diagram of literature screening.
Baseline data analysis and quality evaluation
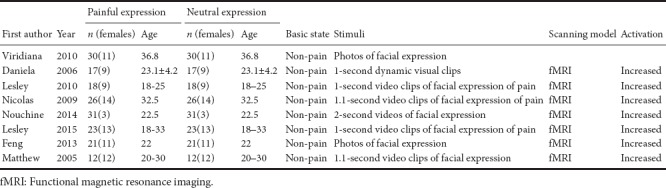
Baseline data and quality evaluation are listed in Table 1.
Table 1.
Basic inclusion criteria for articles
Meta-analysis results
Increased activation in the brain areas under the stimulation of facial expressions of pain (Table 2 and Figure 2) showed 20 foci in eight trials with healthy subjects, including left precentral gyrus (BA44), left inferior frontal gyrus (BA45), bilateral middle temporal gyrus (BA37), right fusiform gyrus (BA37), left thalamus ventral lateral nucleus, bilateral putamen, bilateral amygdala, left cingulate gyrus (BA32), right middle temporal gyrus (BA39), right superior temporal gyrus (BA22), left inferior occipital gyrus (BA18), left middle occipital gyrus (BA18), right caudate body, left inferior occipital gyrus (BA19), right insula (BA13), left middle temporal gyrus (BA21), right middle frontal gyrus (BA46), and left inferior frontal gyrus (BA47). Because the activation in the brain areas of facial expression of pain is affected by age (Martin et al., 2005; Jackson et al., 2006), the results are based on subjects in the young and middle ages ranging from 15–47 years.
Table 2.
Brain areas activated by the stimulation of facial expressions of pain
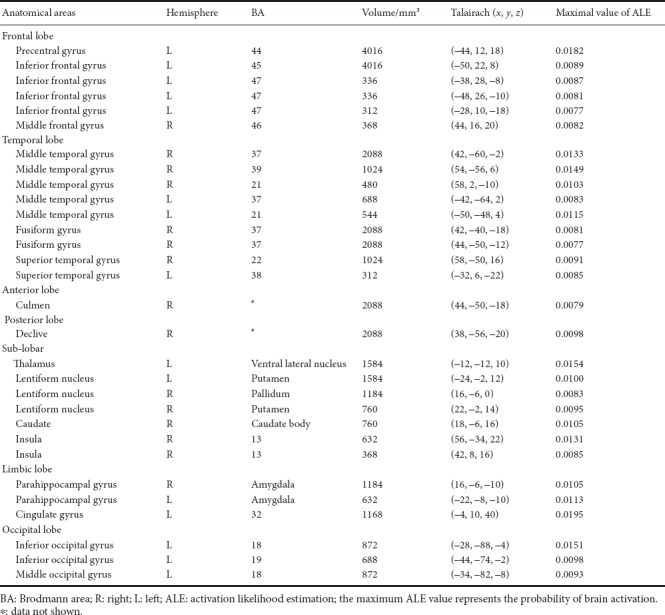
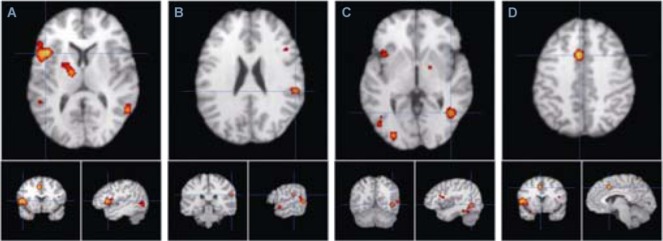
Figure 2.
Main activated brain areas for empathy induced by images of pained facial expressions.
(A–D) The orange zones show that (A) the precentral gyrus (BA 44, −44, 12, 8), (B) the right insula (BA13, 56, −34, 22), (C) the right middle temporal gyrus (BA37, 42, −60, −2) and (D) the left cingulate gyrus (BA32, −4, 10, 40), which were activated under the stimulation of facial expressions of pain. The anterior cingulate cortex (BA32), anterior central gyrus (BA44), fusiform gyrus, and insula (BA13) were activated positively as major brain areas. BA: Brodmann area; (−44, 12, 8), (56, −34, 22), (42, −60, −2), (−4, 10, 40): Montreal Neurological Institute coordinate.
Discussion
Pain is a physiological and psychological activity, including the feeling and response to pain (Li et al., 2018). Empathy refers to the process of experiencing the situation of others, so as to feel and understand the emotions of others. Empathy with pain is the individual’s perception, judgment, and emotional response to the pain of others (Danziger, 2006). Existing studies using picture-based paradigms are inconsistent. We aimed to explore the sympathetic brain regions based on facial expressions from eight eligible studies. In this study, data from eight eligible studies were analyzed using GingerALE 2.3.6, and images were processed with Mango 4.0.1. Our results showed that the fusiform gyrus, cingulate gyrus, precentral gyrus, ventral lateral nucleus, inferior occipital gyrus, middle temporal gyrus, and insula were activated by the sight of a person with a pained expression.
Pained facial expressions cause pain and empathy
Previous studies have shown that the anterior cingulate gyrus and the insula belong to the painful sympathetic core networks (Gu and Han, 2007), which are responsible for the processing of emotional components of the pain experience (Price, 2000). The cingulate gyrus is associated with somatosensory awareness (especially pain perception related to emotional judgment) (Hsieh et al., 1996; Killgore and Yurgelun-Todd, 2007) and the regulation of physical and visceral activities; the anterior insula is associated with emotion, coordinating BA14, BA15, and BA16 to deal with sensory experience and to identify facial expression (Gornotempini et al., 2001). In this study, the cingulate gyrus (BA32) and inferior insula (BA13) were highly activated by the sight of pained expressions, indicating that, as with other experimental paradigms, the painful sympathetic core networks were also activated when watching others’ pained facial expressions. This suggests that simply watching facial expression can activate the pain sympathetic core networks. In addition, the precentral gyrus (BA44) was also highly activated, which may be due to its mirrored neurons (Leube et al., 2001; Lotze et al., 2006). Through this neuronal activity, the observer consciously shows similar expressions or similar brain activities (Rizzolatti and Craighero, 2004; Keysers, 2009). Therefore, some scholars believe that it is because of the mirror neurons that humans have the ability to empathize (Cheng et al., 2008).
Pain empathy and physical pain have a common neural network
By directly stimulating the brain, the activated brain areas generally include the primary sensory cortex (S1), secondary sensory cortex (S2), insula, and anterior cingulate cortex (Apkarian et al., 2005). The lateral thalamus is also involved in sensory formation (Ro et al., 2007). This study found that the brain areas activated by an expression of pain also include the insula, anterior cingulate cortex, and ventral lateral nucleus. A large number of studies have shown that activation by pain is mainly in the anterior cingulate cortex and insula (Jackson et al., 2005, 2006; Ogino et al., 2006; Moriguchi et al., 2007; Morrison and Downing, 2007; Lamm et al., 2011), and these areas overlap with the responses of the brain to a pained expression. We can conclude that body pain and empathy for pain share some common parts of the nerve pathways, which mainly comprise the emotion-related brain areas.
Pain empathy mainly includes an emotional element, which is different from that of physical pain
The sensory components of the pain experience are processed by the primary and secondary somatosensory cortex (S1, S2), which is based on their anatomical structures and their connection with the subcortical region and the spine; thus, they primarily process and identify sensory stimulation of the body (Rainville et al., 1997; Rainville, 2002; Fusar-Poli et al., 2009). Because this study does not involve direct physical pain stimulation, the results do not contain S1 and S2. This reflects that empathy is an emotional experience rather than a somatic sensation. Studies have shown that the secondary sensory cortex and sensory motor cortex are activated only when there is direct stimulation by pain (Singer et al., 2004). These findings also support the findings of this study. However, some studies indicate that even in the absence of actual pain stimuli, the sensorimotor cortex will be activated (Ogino et al., 2006; Gu and Han, 2007). This may result from the differences in research paradigms and stimulus patterns (Lamm et al., 2011). It is noteworthy that some scholars have found that the empathetic brain areas and the emotional components of pain are not exactly the same (Jackson et al., 2006). This may indicate that pain empathy is not equivalent to pain emotional processing.
The brain areas associated with facial recognition and facial expression
The brain regions involved in facial recognition may include the fusiform gyrus, inferior occipital gyrus, anterior central gyrus, and insula (Saarela et al., 2007; Zhen et al., 2013). In this study, the brain area associated with simple face recognition is mainly the fusiform gyrus. The fusiform gyrus is located between the temporal gyrus and the hippocampus, with the ability to recognize a face and body. The region within that responsible for face recognition is called the fusiform face area (Kanwisher et al., 1997; Slotnick and White, 2013; Axelrod and Yovel, 2015). The fusiform face area can produce similar recognition for non-face visual images (McCarthy et al., 1997; Rossion et al., 2000), which is a prerequisite for facial perception and is associated with the posterior temporal gyrus (BA21), both belonging to the visual cortex. However, the fusiform gyrus is not only activated under the stimulation of facial expressions; although the fusiform face area is a part of the fusiform gyrus and is responsible for face recognition, the fusiform gyrus has further functions, such as processing information on non-facial stimulation. Studies have shown that the fusiform gyrus is also activated in subjects who are watching others’ hands and feet (Eidelman-Rothman et al., 2016), indicating that it is widely involved in general recognition.
Facial expression recognition and general simple visual stimulation are different
Investigating studies using the picture paradigm, this analysis is bound to find data involving the activation of visual pathways (Deyo et al., 2004).
The suboccipital region (BA18) is the secondary visual cortex, and together with BA19 constitutes the visual cortex. However, the visual cortex was not highly activated. This indicates that during pain empathy caused by watching a pained expression, the production of visual information is only a relatively minor stage. Combined with the fusiform gyrus, which is highly activated, it can be seen that the recognition and processing of faces and facial expressions are the leading steps: in other words, the activation of the brain area involved in facial recognition distinguishes stimulation by facial expression from stimulation by nonfacial images.
From the results of our study, the occipital lobe, the frontal lobe, the temporal lobe, and the limbic system were differently and strongly activated. Based on the existing studies and combined with this meta-analysis, we can make assumptions that an image of a pained facial expression produces a pain sympathetic activation of the brain regions as follows:
The pained facial expression image can stimulate the emotional network, which produces spontaneous emotion processing. The image activates the occipital lobe first, followed by the temporal lobe, the insula, the limbic system, and other emotional networks. Among them, the frontal lobe processes the emotional stimulation. In other words, the frontal lobe processes awareness through the integration of physical and subjective feelings and objective information (Osborn and Derbyshire, 2010).
In addition, the human brain will produce direct emotions compared with the self’s existing emotional experience, and will then recognize the emotions caused directly by some certain stimuli and find the part that belongs to the empathetic feelings. The prefrontal cortex, hippocampus, and other brain areas related to memory and cognition were activated after the emotional network was activated. The cingulate gyrus, hippocampal gyrus, and other parts of the pain sympathetic core network are strongly activated, and then the empathy for pain occurs (Zhou et al., 2004).
The limitations of this study are as follows: (1) Despite repeated searches and manual review, the number of included studies is still insufficient: the sample capacity is small, and can easily cause statistical error. (2) Parts of the studies did not test the subjects’ ability for empathy (Mazza et al., 2015), leading to a decline in the quality of this article. (3) Because of an insufficient number of articles, we did not divide the stimulating methods into two groups (static pictures and dynamic short videos), and we did not distinguish between a first-person perspective (imagining that you are suffering the same painful experience as a character in the picture) and a third-person perspective (imagine yourself witnessing the suffering of the characters in the picture)..
In conclusion, the ALE analysis showed that the anterior cingulate cortex, insula, anterior central gyrus (BA44), and fusiform gyrus were activated positively in response to images of pained facial expressions. Pained facial expressions activated the brain regions related to pain empathy, including areas of the emotional circuit and facial recognition, further contributing to revealing the brain’s mechanisms of pain empathy.
Additional file: Open peer review report 1 (46.9KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81473769 (to WW), 81772430; a grant from the Training Program of Innovation and Entrepreneurship for Undergraduates of Southern Medical University of Guangdong Province of China in 2016, No. 201612121057 (to WW). The conception, design, execution, and analysis of this manuscript, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Reporting statement: This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Chaur-Jong Hu, Taipei Medical University, China.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81473769 (to WW), 81772430 (to WW); a grant from the Training Program of Innovation and Entrepreneurship for Undergraduates of Southern Medical University of Guangdong Province of China in 2016, No. 201612121057 (to WW).
P-Reviewer: Hu CJ; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Cone L, de Souza M, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Apkarian AV, Bushnell MC, Treede R, Zubieta J. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod U, Yovel G. Successful decoding of famous faces in the fusiform face area. PLoS One. 2015;10:e0117126. doi: 10.1371/journal.pone.0117126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Budell L, Jackson PP. Brain responses to facial expressions of pain: Emotional or motor mirroring? Neuroimage. 2010;53:355–363. doi: 10.1016/j.neuroimage.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Budell L, Kunz M, Jackson PL, Rainville P. Mirroring pain in the brain: emotional expression versus motor imitation. PLoS One. 2015;10:e107526. doi: 10.1371/journal.pone.0107526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Yang C, Lin C, Lee P, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage. 2008;40:1833–1840. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 7.Danziger N. Is pain the price of empathy? The perception of others’ pain in patients with congenital insensitivity to pain. Brain. 2006;129:2494–2507. doi: 10.1093/brain/awl155. [DOI] [PubMed] [Google Scholar]
- 8.Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 10.Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Deyo KS, Prkachin KM, Mercer SR. Development of sensitivity to facial expression of pain. Pain. 2004;107:16–21. doi: 10.1016/s0304-3959(03)00263-x. [DOI] [PubMed] [Google Scholar]
- 12.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eidelman-Rothman M, Goldstein A, Weisman O, Schneiderman I, Zagoory-Sharon O, Decety J, Feldman R. Prior exposure to extreme pain alters neural response to pain in others. Cogn Affect Behav Neurosci. 2016;16:662–671. doi: 10.3758/s13415-016-0422-7. [DOI] [PubMed] [Google Scholar]
- 14.Enzi B, Amirie S, Brune M. Empathy for pain-related dorsolateral prefrontal activity is modulated by angry face perception. Exp Brain Res. 2016;234:3335–3345. doi: 10.1007/s00221-016-4731-4. [DOI] [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 16.Gornotempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umità C, Nichelli P. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 17.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Gu X, Han S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav Brain Res. 2007;181:218–223. doi: 10.1016/j.bbr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Hadjikhani N, Zürcher NR, Rogier O, Hippolyte L, Lemonnier E, Ruest T, Ward N, Lassalle A, Gillberg N, Billstedt E. Emotional contagion for pain is intact in autism spectrum disorders. Transl Psychiatry. 2014;4:e343. doi: 10.1038/tp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh JC, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain. 1996;67:59–68. doi: 10.1016/0304-3959(96)03066-7. [DOI] [PubMed] [Google Scholar]
- 21.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Kam JW, Xu J, Handy TC. I don’t feel your pain (as much): the desensitizing effect of mind wandering on the perception of others’ discomfort. Cogn Affect Behav Neurosci. 2014;14:286–296. doi: 10.3758/s13415-013-0197-z. [DOI] [PubMed] [Google Scholar]
- 24.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keysers C. Mirror neurons. Curr Biol. 2009;21:R971–R973. doi: 10.1016/j.cub.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Killgore WD, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Soc Neurosci. 2007;2:28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- 27.Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport. 2001;12:2773–2777. doi: 10.1097/00001756-200108280-00035. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Feng C, Ning M, Xu F, Song ZH, Huang ZB, Xiao HY. Angiotensin II receptor antagonist EMA401 used for sciatic nerve constriction-induced neuropathic pain in rats: behavior assessment and anaIgesic mechanisms. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:1909–1914. [Google Scholar]
- 31.Loggia ML, Mogil JS, Bushnell MC. Empathy hurts: compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136:168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Lotze M, Heymans U, Birbaumer N, Veit R, Erb M, Flor H, Halsband U. Differential cerebral activation during observation of expressive gestures and motor acts. Neuropsychologia. 2006;44:1787–1795. doi: 10.1016/j.neuropsychologia.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Martin R, Williams J, Hadjistavropoulos T, Hadjistavropoulos HD, MacLean M. A qualitative investigation of seniors’ and caregivers’ views on pain assessment and management. Can J Nurs Res. 2005;37:142. [PubMed] [Google Scholar]
- 34.Mazza M, Tempesta D, Pino MC, Nigri A, Catalucci A, Guadagni V, Gallucci M, Iaria G, Ferrara M. Neural activity related to cognitive and emotional empathy in post-traumatic stress disorder. Behav Brain Res. 2015;282:37–45. doi: 10.1016/j.bbr.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J Cogn Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- 36.Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Empathy and judging other's pain: an fMRI study of alexithymia. Cereb Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- 37.Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 38.Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2006;17:1139–1146. doi: 10.1093/cercor/bhl023. [DOI] [PubMed] [Google Scholar]
- 39.Osborn J, Derbyshire SW. Pain sensation evoked by observing injury in others. Pain. 2010;148:268–274. doi: 10.1016/j.pain.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 41.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 42.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 43.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 44.Ro T, Farnè A, Johnson RM, Wedeen V, Chu Z, Wang ZJ, Hunter JV, Beauchamp MS. Feeling sounds after a thalamic lesion. Ann Neurol. 2007;62:433–441. doi: 10.1002/ana.21219. [DOI] [PubMed] [Google Scholar]
- 45.Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, De Gelder B, Zoontjes R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci. 2000;12:793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- 46.Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cereb Cortex. 2007;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- 47.Sheng F, Liu Q, Li H, Fang F, Han S. Task modulations of racial bias in neural responses to others’ suffering. Biol Psychol. 2013;92:380–386. doi: 10.1016/j.biopsycho.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Simon D, Craig KD, Miltner WHR, Rainville P. Brain responses to dynamic facial expressions of pain. Pain. 2007;126:309–318. doi: 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Simon D, Craig KD, Gosselin F, Belin P, Rainville P. Recognition and discrimination of prototypical dynamic expressions of pain and emotions. Pain. 2008;135:55–64. doi: 10.1016/j.pain.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Singer T, Lamm C. The social neuroscience of empathy. Ann NY Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 51.Singer T, Seymour B, O J, Doherty, Kaube H, Dolan RJ. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 52.Slotnick SD, White RC. The fusiform face area responds equivalently to faces and abstract shapes in the left and central visual fields. Neuroimage. 2013;83:408–417. doi: 10.1016/j.neuroimage.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 53.Turkeltaub P, Eden G, Jones K, Zeffiro T. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 54.Vachon-Presseau E, Roy M, Martel MO, Albouy G, Chen J, Budell L, Sullivan MJ, Jackson PL, Rainville P. Neural processing of sensory emotional-communication information associated with the perception of vicarious pain. Neuroimage. 2012;63:54–62. doi: 10.1016/j.neuroimage.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 55.Zhen Z, Fang H, Liu J. The hierarchical brain network for face recognition. PLoS One. 2013;8:e59886. doi: 10.1371/journal.pone.0059886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou S, Zhou W, Chen X. Spatiotemporal analysis of ERP during Chinese idiom comprehension. Brain Topogr. 2004;17:27–37. doi: 10.1023/b:brat.0000047334.48256.9f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.